In this article we will discuss about the procedure for obtaining fungal culture in the pure form.
The mycologist raises pure culture of a fungus to study the fungus in detail regarding its reproduction, physiology and genetics.
The pure culture may be on liquid solid/media and in obtaining the pure culture of the organism, the mycologist employs certain procedure.
1. Preparation of Culture-Media:
Cleaning of Glass wares:
All glass wares used in microbiological or pathological studies should be rigidly cleaned. Initial cleaning should be done with soap or vim using a brush.
They can finally be washed with the following mixture:
The same mixture can be used several times. After washing with this mixture, the glasswares are washed for sometimes in running tap water, then finally with distilled water and allowed to air dry.
Preparation of Media:
The choice of medium depends on the type of microorganism to be grown taking into consideration the nutritional requirements of the particular form.
Out of a large number of such media, some are described below:
(i) Based on the consistency, the culture media are of two types:
(a) Liquid media or Broth:
These media are liquid in consistency and are used in cases where microbial growth in pure form is to be separated out for further studies.
(b) Solid Media:
The liquid media can be solidified by the addition of Agar-Agar, a jelly like substance extracted from the sea-weed, Gelidium. Addtion of about 1.5% of this substance at or near the neutral pH is sufficient to make the medium solid.
(ii) Based on the composition, the culture media may be of two types:
(a) Natural Media:
These media incorporate certain naturally available substances of “Somewhat” unknown composition. Some examples of such media are: Malt extract agar. Soil extract agar. Oat meal agar and Potato dextrose agar.
Potato dextrose agar is commonly used for phytopathological studies.
The composition of Potato Dextrose agar (PDA) is given below:
Potato slices are allowed to simmer in 500 ml. distilled water, the extract filtered by means of a muslin cloth, dextrose and agar-agar powder are added, the volume made up to 1000 ml and made to boil.
(b) Synthetic Media:
These media have known constituents.
Examples of synthetic media are Standard synthetic and Czapek-Dox media, composition of which are given below:
All the constituents are weighed out distilled water added and the mixture heated to boiling. It will be better to add K2HPO4 or KH2PO4 in the end to avoid precipitation. The pH is adjusted as desired by adding NaOH or Citric acid solutions in required quantity.
Filtration of Media and dispensing into culture tubes or Flask:
To a large funnel, a rubber tubing with a stop-cock is fitted. The medium is filtered through a piece of muslin cloth and the appropriate quantities are dispensed into culture tubes (6″x 3/4″) or flasks (250 ml capacity), by adjusting the stop-cock.
All the glasswares in which the medium it is to be dispensed must be plugged with cotton wool. Culture tubes meant to be agar slants should receive approximately 10 ml of the medium while those ultimately to be used for plating (in flasks) approximately 50ml. The remaining medium should be put in flasks, plugged with cotton wool and used as required.
Sterilization of the Medium: Autoclaving:
The media thus dispensed with should be steam-sterilized in an autoclave for 15 mts at 16lbs/inch pressure. For convenience, the culture tubes are arranged in a wire basket and then put in the autoclave. Before opening the autoclave, the pressure is allowed to come down to zero.
Slanting:
The culture tubes with 10 ml of medium and meant for use as tube slants should be taken out from the autoclave while hot, slightly rotated, put on a slanting position on a slanting rack and allowed to cool.
The medium due to the presence of agar-agar would solidify on cooling. The agar slants are now ready for culture work.
Sterilization of Glass-ware:
It may be done in 2-ways.
1. Dry heat sterilization:
This involves heating the glasswares (especially petridishes meant for planting work) in ‘an electric oven at 150°C for 30 mts. The petridishes in suitable batches should be wrapped in bamboo paper and tied with a thread and then placed in the oven.
2. Steam sterilization:
For certain type of culture work, this method should be used. The glassware should be plugged with cotton wool and steam sterilized in an autoclave at 15Ibs/inch pressure for 15 mts.
Plating:
It should be done in a culture room sterilised through sulphur or formaline fumigation. The table on which the process is to be carried out should be cleaned with a swab of cotton dipped in alcohol.
All these steps will help to check the contamination of plates by unwanted microorganisms. The medium in the flask should be used for plating. If the medium in the flasks has solidified, it has to be melted in a water bath.
The flasks should be taken to a culture room, the cotton plug opened, the mouth gently heated on a flame, the upper lid of the petridish slightly raised, the medium is poured into the pertridish, the lid replaced and the medium allowed to cool.
The process of plating the medium should be carried out in as little time as possible in order to avoid any contamination. Now, the petridishes with the culture medium are ready for inoculation with the microorganism.
2. Isolation of Micro-Organisms from Soil:
Various methods have been devised to isolate pure cultures of microorganisms from soil.
Methods which yield a relatively broad spectrum of microorganisms are commonly used by plant-pathologists for isolating soil borne pathogens and are described here:
1. Direct inoculation:
A crumb of soil, about 1cm in diameter is transferred with a sterilized forceps to the centre of a sterile petridish containing solidified agar medium. The petri-dish is incubated at 28-30°c and the mycelia radiating out from soil crumb are cut and transferred to agar slants.
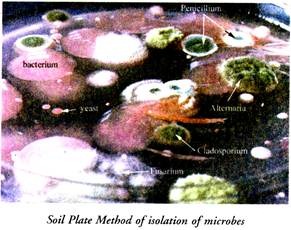
2. Soil-Plate Method:
A small amount of soil is transferred into a sterile petridish with the help of a sterilized spatula. 20 ml of melted and cooled agar medium is added to the petridish containing soil and the pertridish is gently shaken so that the soil-particles are dispersed throughout the agar medium.
The petridish thus obtained is incubated at 28°-30°C for a few days after which the fungi begin the appear. Pure cultures of the desired fungus is obtained by transferring that particular fungus to agar slants.
3. Soil-dilution Plate Methods:
10 gms. of soil sample is taken in a 250 ml conical flask. To this is added 100 ml of sterile water and the flask is vigorously shaken for a few minutes so that soil solution is obtained.
This will represent 1:10 or 1/10. 10ml of the supenatent of this 1/10 solution is taken and 90 ml of sterile water is added and the resultant solution will be 1/100. In the same way, other dilutions like 1/1000, 1/10,000 and 1/100000 are made.
One ml of the desired dilution is poured into a sterile petri-dish and to this is added 20 ml of melted and cooled agar medium. The petri-dish is rotated by hand to disperse the medium and the soil suspension.
The petri-dishes are then incubated at 28°-30°C for a few days after which the growth of fungi takes place. Pure cultures of the fungi are then obtained by transferring them to agar slants.
Fungal culture of pure form is obtained by inoculating a single spore or a piece of mycelium on the agar medium in tube slants or in petridishes as shown in Fig. 17.11. The inoculated agar plate is covered and the tube slant is plugged with non- absorbent cotton wool.
They are then placed in an incubator which is usually maintained at a temperature of 30-37°C for a couple of days after which the agar plates and slants will show fungal growth in pure form.