In this article we will discuss about the life cycle of puccinia graminis with the help of suitable diagrams.
Dikaryophase in the Life Cycle of Puccinia:
This phase in the life cycle of Puccinia is confined to the primary host which is wheat. It consists of dikaryotic mycelium and two spore stages, uredineal and telial.
1. Dikaryotic Mycelium:
It is internal and thus invisible until it is ready to reproduce.The binucleate secondary or dikaryotic mucelium is filamentous, well developed and branched.
The hyphal branches, which are septate, ramify in the intercellular spaces of the tissues of the stem and leaves of the host plant (wheat Fig. 14.14 B). The septal pore is simple. Each cell contains a pair of nuclei (n+n) constituting a dikaryon.
The nuclear membrane is double layered and perforated. Besides the two nuclei, the cytoplasm contains free ribosomes mitochondria, glycogen particles, lipid bodies and other unidentified particles.
To obtain nutrition the intercellular hyphae develop intracellular food absorbing organs called haustoria.
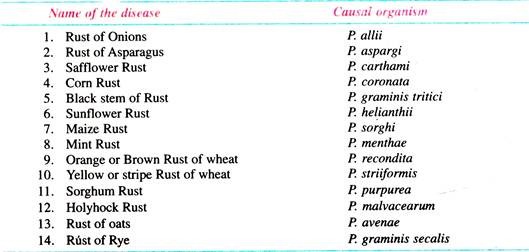
List of important diseases caused by species of Puccinia:
Haustorial Apparatus:
Allen (1923) reported that the tips of certain hyphae of the intercellular dikaryotic mycelium come in contact with the cell walls of the host cells and become separated from the remainder of the mycelium by septa.
The resultant small, binucleate tip cells swell somewhat and function as haustorial mother cells. At the point of contact, each haustorial mother cell develops a buldge-like thickening which is closely appressed to the host cell wall.
A fine pore appears at the point of contact extending through the cell walls of both the pathogen and the host. A delicate infection peg from the haustorial mother cell passes into the host cell through the pore.
After penetration, the infection peg elongates to form the haustorial neck. A wall is formed around it by the fungal pathogen. The plasma membrane of the host invaginates as the developing neck elongates.
The latter eventually ceases to elongate. Its apical portion expands to form the body region of the haustorium. Dickinson (1949, 1955) stated that the formation of haustoria by the infection hyphae is induced by contact stimulus.
The intercellular mycelium probably by enzymatic degradation enters the host cells. The haustoria may be knob-like or finger-like, rarely convoluted. Rykenberg and Truter (1973) reported that the intracellular saccate or extensively lobed body of the haustorium is connected by a narrow penetration tube or the stalk to the extracellular haustorial mother cell.
The hyphal wall and the plasma membrane of the haustorial mother cell are continuous along the entire length of the penetration tube and haustorial body constituting the haustorial wall and haustorial membrane respectively.
A collar-like structure attached to the host cell wall and made of the same material is produced by host cell in response to the presence of the penetration tube. It surrounds the penetration tube at its proximal end.
The haustorial body is binucleate. On the outer face of the hyphal wall (or haustorial wall) in the body region only is formed an electron dense sheath matrix consisting of a granular amorphous material.
The sheath matrix is also designated as the extrahaustorial sheath. Since the fungus obtains nutrition from the host the infected wheat plants exhibit stunted growth.
Despite the serious damage that the rust causes the host plant never succumbs completely. The yield of wheat crop is, however, reduced. Each cell of the fungus mycelium has a pair of nuclei (dikaryon).
The transverse septa between the cells have each a central pore without a dolipore parenthesome complex. The clamp connections, however, have not been reported on the dikaryotic mycelium.
The mycelium is generally localised. It grows and ramifies near the point of infection extending only short distance into the host tissue. The fully developed dikaryotic mycelium enters upon the reproductive phase.
Reproduction in Puccinia Graminis:
The dikaryotic mycelium of Puccinia graminis reproduces by sporulation. The spores produced are of two kinds, the uredospores and the teleutospores or teliospores.
They are produced near the surface of the host tissues. When mature they break through in slits or pustules called the sori. The mature spores are thus seen externally.
(a) Uredineal Stage (Fig. 14.14):
Prior to uredospore formation, the hyphae of the dikaryotic mycelium begin to aggregate near the surface of the infected organ such as stem, leaf, leaf sheaths or glumes to form a hyphal mass which surrounds isolated host cells (B).
The mycelial hyphae then produce masses of cells subepidermally. These are called uredia. From the uredia arise vertically growing slender, stalk-like hyphae arranged in a close palisade-like layer.
The tip of each hyphal stalk or uredospore mother cell swells to form a single binucleate oval uredospore or uredeniospore. The uredospores are thus formed in groups. Each such group is called a uredosorus or uredinium (B).
The developing uredosori are seen in the wheat leaf as pale streaks. They exert pressure on the overlying epidermis which is, at first, lifted but finally ruptured in the form of slits or blisters (A).
It is through these slits that the rusty coloured masses of uredospores are seen externally. They are stalked, unicellular, oval, binucleate structures (C). In masses the uredospores appear rusty red in colour.
The uredospore wall is thick. It is differentiated into three layers: outermost pellicle the middle exosporium and the inner thin endosporium.
The outermost layer (pellicle) is faintly echinulate and generally with four equatorially arranged thin areas on it. These thin areas on the otherwise thick wall are called the germ pores.
Harder et al (1986) however, consider the uredospore wall to probably be composed of 5 layers including the pellicle as the outermost layer. The uredospore wall contains silica deposits.
The binucleate uredospores function as conidia and are capable of germination as soon as they are produced. They are carried in the air to healthy wheat plants which they infect immediately.
The uredospores thus serve as dispersal agents of rust rapidly multiplying the dikaryophase during the growing season. However, unlike the conidia of Ascomycetes, the uredospores are binucleate. They are able to infect wheat plants only and not the alternate host barberry.
Germination of Uredospores and infection of the host (Fig. 14.15 B-C):
On falling on another wheat plant and under suitable conditions the uredospore germinates within a few hours. The endosporium comes out in the form of a slender tube through the germ pore (B).
More than one germ tubes may be produced by the same uredospore. They emerge through different germ pores.
The germ tube by elongation grows over the surface of the host leaf till it reaches a stoma where its tip swells to form an appresorium (C). The two nuclei and the protoplasm of the germ tube migrate into the appresorium.
The appresorium is then cut off from the empty germ tube by a septum. Both the germ tubes and the appresoria walls are composed of 2 layers and appear to be coated with mucilaginous-like substance.
A peg-like outgrowth, the infection peg arises from the centre of the free end of the appresorium. It enters the stomatal aperture. Reaching the substomatal cavity the tip of the infection peg swells up into a vesicle.
The contents of the appresorium pass through the infection peg into the sub-stomatal vesicle. Infection hyphae, one or more in number, then develop from the vesicles, grow towards the neighbouring host cells and ramify in the intercellular spaces between them.
The hyphae grow vigorously and form the new dikaryotic mycelium between the mesophyll cells of the host tissue. In a few days time the new mycelium produces a new crop of uredospores.
This repeated cycle recurs several times in a single growing season. As a result the disease spreads in a few days from plant to plant and field to field over a large area provided the environmental conditions are favourable.
Temperature and humidity are the two climatic factors which facilitate infection and thereafter the development of the dikaryotic mycelium of black stem rust of wheat.
The source of hymidity may be the dew drops, guttation drop, light rainfall or irrigation water. In a thin film of water and light intensity below 300 ft C, the optimum temperature for germination ranges between 15-24°C for penetration and substomatal vesicle formation 29°C and germ tube growth 20°C.
For maximum infection a definite sequence of temperature, humidity and sunlight is required.
(b) Telial Stage (Fig. 14.16):
Late in the growing season another kind of spore begins to develop from the uredia in the uredosori. It is the teleutospore. The teleutospores are, at first, developed among the uredospores in the same sorus (Fig. 14.14 A). They are of dark brown or black colour.
Gradually as the season progresses more and more teleutospores are produced whereas the number of uredospores is reduced. Finally the sori contain only the teleutospores (B).
These sori are called the teleutosori. The teleutospores in the teleutosorus exert pressure on the overlying epidermis which is at first lifted but finally ruptured. The black teleutospores are exposed (A).
The cells of the mycelium producing the teleutospores are called the telia. This stage in the life cycle in which the teleutospores are produced is called the telial stage or the black spore stage.
It is considered the perfect stage of the Uredinales because it is in the teleutospores that karyogamy and meiosis take place.
The teleutospores (Fig. 14.16 C) are black or dark brown, stalked, two-celled, spindle-shaped structures slightly constricted at the septum.
The black spore wall is thick and smooth. The free end of the spore may be rounded to slightly pointed with a thick wall at the apex. There is a single germ pore in the wall of each cell.
It is at the apex in the upper cell of the spore and a little below the septum in the lower cell. Each cell of the teleutospore has two nuclei (one of plus strain and the other of minus strain).
Unlike the uredospores, the teleutospores are borne on persistent pedicels and do not germinate immediately.
During maturation period of teleutospore, karyogamy takes place. The two nuclei in each cell of teleutospore fuse to form a diploid nucleus.
Overwintering takes place in the uninucleate diploid condition of the bicelled teleutospore which represents the reduced diplophase in the life cycle.
After the harvesting period, mature teleutospores remain dormant on straw, stubble and out of season self-grown plants in the fields and survive even the severest winters. After the resting period the teleutospore germinates if the conditions are favourable.
The conditions favourable for their germination are high atmospheric humidity, presence of moisture and freezing condition prior to germination. The teleutospores germinate to give rise to the basidial stage in the life cycle of Puccinia.
Basidial Stage (Fig. 14.17):
After the resting period and under favourable conditions the teleutospores germinates in situ to produce the basidial stage in the life cycle. The characteristic structures of this stage are the basidia and the basidiospores.
The basidium in Puccinia is divisible into three parts namely:
(i) Probasidium or hypobasidium
(ii) The epibasidium or metabasidium and
(iii) The sterigmata which bear the basidiospores.
Each cell of the teleutospore containing a diploid nucleus represents the probasidium or hypobasidium. At the time of germination a short, slender hyaline hypha of limited growth, the promycelium or epibasidium grows out through the germ pore from each cell (probasidium) of the teleutospore (C).
This is followed by the migration of the diploid nucleus into the epibasidium. There the diploid nucleus undergoes two divisions constituting meiosis (C, a). Segregation of strains takes place.
The four haploid nuclei thus produced in each epibasidium come to lie at more or less equal distances. Septa appear between the nuclei dividing the epibasidium into four uninucleate haploid cells (C, b).
From each of the three lower epibasidial cells then grows a short narrow tube, the sterigma. The sterigma usually arises from just below the upper septum of the cell. From the terminal cell of the epibasidium, the sterigma arises from the apex.
The free tip of each sterigma swells to form a basidiospore (D, a). In the meanwhile the haploid nucleus from each epibasidial cell passes up its respective sterigma into the developing basidiospore.
Two out of the four basidiospores on each epibasidium are of plus strain and the other two of minus strain. They are small, nuicellular, uninucleate haploid structures (D, b).
When mature the basidiospores are discharged by the water droplet method or spore drop mechanism with a force into the air. They are carried by wind to the leaves of alternate host barberry which they infect.
The basidiospores remain viable only for a few days. They cannot bring about infection of wheat and thus perish soon if the suitable (alternate) host is not available.
Haplophase in the Life Cycle of Puccinia Graminis:
This phase in the life cycle is confined to the alternate host Barberry (Berberis vulguris). It starts with the basidiospores.
The haplophase consists of primary or haplomycelium, and the two spore stages. These are the spermagonial (also called pycnidial) and aecial (aecidial) stages (Fig. 14.22 A).
1. Haplomycelium:
The basidiospore germinates (Fig. 14.24) on the leaf of the alternate host (Berberis) provided the moisture and temperature conditions are suitable. It puts out a germ tube also called the primary hypha.
The primary hypha infects the young leaf of the alternate host by piercing through the epidermis directly. The cuticle of older leaves is too thick to allow penetration by the delicate germ tube.
Once within the host tissue it grows vigorously and branches freely to form the primary mycelium, also called monokaryotic or haplomycelium. The primary mycelium is septate. Its component cells are uninucleate.
The mycelial hyphae ramify in the intercellular spaces between the mesophyll cells of the leaf. They produce haustoria which penetrate the cells of the host tissue and obtain nutrition for the growth of the mycelium.
It may often happen that several basidiospores of different strains infect the same Barberis leaf.
Naturally the newly formed haplomycelia will be of two different strains (plus and minus). They develop side by side. The two strains of mycelia can thus co-exist in the same leaf.
Even if they are very close to each other no fusion occurs between them in their early stages of development. Consequently each mycelium remains haploid for some time. Fusion may occur later.
2. Spermagonial Stage (Fig. 14.18):
About four days after infection the hyphae of the haplomycelium begin to collect beneath the upper epidermis. They form a dense mat. Small light patches on the upper surface of the barberry leaf are the external signs of infection at this stage.
From this mycelial mat arise groups of hyphae which fashion into small, flask-shaped structures (Fig. 14.18) called spermagonia (also called the pycnidia). The pycnidia or
spermagonia, like the mycelia from which they arise, are of plus or minus strain.
When spermagonia mature, the infected areas becomes swollen and are seen as small, orange yellow bumps on the
upper surface of infected leaf. The spermagonia are sub-epidermal in position.
They are buried in the mesophyll tissue of the area which gets thickened as a result of the presence of the fungus mycelium.
Each spermagonium (Fig. 14.18) consists of a wall surrounding a cavity. It opens on the upper surface of the host leaf through a small pore called an ostiole.
From the wall of the spermagonium arise three kinds of hyphae:
(i) Spermatiophores:
Numerous fine, elongated, uninucleate cells or short hyphae arise from the cells of the wall (Fig. 14.18). They project into the cavity of the spermagonium and are called the spermatiophores.
They are closely packed and arranged in a palisade-like layer lining the cavity. Each spermatiophore (Fig. 14.19) by successive divisions of its nucleus abstricts at its free tip a number of small cells one after the other.
These are the spermatia. Each spermatium has a single nucleus and very little cytoplasm. The mature spermatia fall into the spermagonial cavity. They are non-motile and cannot infect either host.
(ii) Periphysis (Fig, 14.18):
They are long, delicate sterile hyphae which develop near the ostiole from the spermagonial wall. At first all the periphyses converge towards a central point.
From there they curve upwards in a cluster towards the ostiole. Finally they project through and beyond the ostiole end.
(iii) Flexuous receptive hyphae (Fig. 14.18):
Adjacent to the ostiole and below or among the stiffer tapering periphyses, develop another kind of hyphae. They are slender, delicate, cylindrical with blunt free tips.
Being flexuous these are named the receptive or flexuous hyphae. They are septate and may be simple or branched. Each septum has a central pore.
The nature of spermagonia and spermatia has long been a debatable point. It was in 1927 that Craigie, Buller and their associates demonstrated that spermatia function as male cells.
The receptive or flexuous hyphae are supposed to function as trichogyne hyphae. They develop late and are spermatised by the spermatia of opposite strain.
Spermatisation (Fig. 14.20):
The mechanism of plasmogamy in Puccinia is known as spermatisation. The mature spermatia exude from the ostiole of spermagonium in a drop of sticky, thick liquid called nectar.
The nectar with its scent and sugary content attracts the insects particularly the flies, to the leaf. The spermatia stick to the legs and proboscis of the visiting insects and thus are dispersed from leaf to leaf or from one spermagonium to another on the same leaf.
By the time the drop of nectar exudes, the receptive hyphae grow out through the ostiole into the nectar. These are spermatised by spermatia of opposite strain brought there by visiting insects (A).
The intervening walls at the point of contact between the spermatium of one strain and the receptive hypha of another strain dissolve.
The spermatium nucleus passes through the opening into the receptive hypha in which it moves downwards through the pores in the septa.
Finally the spermatial nucleus reaches the basal cell of the receptive hypha which thus comes to possess two nuclei which lie side by side in a pair (C). One of these nuclei is of minus strain and the other of plus strain.
This pair of nuclei of opposite strain is called a dikaryon. The cell possessing the dikaryon is said to be diploidised. As a result of spermatisation, the basal cells of one or more receptive hyphae in the spermagonium become dikaryotised.
From this account it is evident that the spermatia and the receptive hyphae have taken over the sole function of sexual reproduction in which plasmogamy takes place by spermatisation.
Dikaryotisation may also occur when haploid hyphae of two mycelia of opposite strain come into contact.
3. Aecidial or Aecial Stage (Fig. 14.21 A-B):
The haplomycelium, which has built up spermagonia beneath the upper epidermis, has in the meanwhile penetrated the entire leaf and reached its lower surface. There the mycelial hyphae accumulate and form spherical masses of uninucleate cells.
These are called the aecidial or aecial primordia. The aecidial primordia or protoaecidia lie singly in the substomatal chambers or in the intercellular spaces beneath the lower epidermis just opposite the spermagonia on the upper surface.
The basal cells of aecidial cups on the lower surface; B, The same enlarged surface. The basal cells of aecidial primordia become binucleate. How this takes place is not clear.
The most prevalent view is that the spermatial nuclei from the basal cells of the receptive hyphae pass into the wall cells of the spermagonium (pycnidium). From there they migrate into the hyphae of the haplomycelium.
In the mycelial hyphae they travel passing down through the septal perforations. Finally these spermatial nuclei reach the cells of the basal layer of the aecial primordia which thus become binucleate.
These binucleate cells of the basal layer are called the aecidiospore mother cells. They divide producing alternately aecidiospores and intercalary cells (Fig. 14.22 B).
Development of aecidiospores (Fig. 14.22 B):
At the time of aecidiospore formation, the binucelate aecidiospore mother cell increases in length. The two nuclei in it divide conjugately. A small daughter cell is then cut off at its terminal end.
Two of the nuclei remain in the aecidiospore mother cell and the other two pass into the daughter cell. The daughter cell increases in size and divides into two, upper bigger binucleate aecidiospore or aeciospore cell and the lower smaller binucleate sterile disjunctor or intercalary cell.
Each aecidiospore mother cell undergoes a series of such divisions. In this way closely packed chains of cells are formed at the tips of the aecidiospore mother cells.
The oldest cell is at the top of the chain and the youngest adjacent to the tip of the aecidiospore mother cell.
In the meantime the marginal cells at the base of the young aecidium divide repeatedly to form a wall which completely surrounds the cells in the aecidium. This wall is called the peridium.
As the aecidia mature the chains of cells grow up. As a result of this, pressure is exerted from within. At first the overlying lower epidermis ruptures and then the aecidium splits open to form a cup-like structure (Fig. 14.22 A).
The aecidial cup is partly within the leaf tissue and partly projects above it. The torn roof of the peridium often forms a lip around the edge of the aecidial cup. By this time the intercalary cells have disorganised.
The aecidiospores in the chains become separated from each other and fill up the aecidial cup (Fig. 14.22 A). The aecidiospores filling the cup are now exposed.
They are, at first, polyhedral, thin-walled, unicellular binucleate structures which are orange coloured. The polyhedral spores absorb water, round off suddenly and thus are jerked out of the aecidium to be disseminated by the wind.
The epispore may be smooth or echinulate and has one or more germ pores. The aecidiospores are unable to germinate on the host (Barberry) on which they are produced.
Germination of Aecidiospore:
The binucleate aecidiospores are carried by air currents to the primary host (wheat) on which they germinate under suitable conditions.
At the time of germination the aecidiospore puts out a germ tube or primary hypha which emerges through the germ pore. The germ tube crawls over the surface of the wheat leaf and finds its way into the host generally through a stoma.
On reaching the stoma the tip of the germ tube swells up to form an appresorium and a vesicle in the substomatal cavity. From the vesicle arise hyphae which grow intercellularly.
They branch and form an extensive dikaryotic mycelium. It is chiefly intercellular. Only haustoria enter the host cells. The mycelium soon begins to form masses of uredospores and repeats the life cycle.
Life Cycle Pattern of Puccinia Graminis:
It is evident from the account given in the preceding pages that five different types of spores are produced in a single cycle of Puccinia.
These are:
1. Spermatia (uninucleate) in spermagonia on Barberry.
2. Aecidiospores (binucleate) in aecidia on Barbarry.
3. Uredospores (binucleate) on the dikaryotic mycelium on wheat.
4. Teleutospores (binucleate) on the dikaryotic mycelium on wheat.
5. Basidiospores (uninucleate) on epibasidia.
The rusts thus have a polymorphic life cycle because of the presence of different types of spores in the life cycle. These spore forms are produced in a regular sequence. The sequence is controlled by inherent internal factors.
It is never reversed. Because of this long life cycle the rust, in which all the five spore stages are produced, is called macrocyclic or long cycled rust. The best example of this is Puccinia graminis tritici.
There are, however, examples of rusts in which the life cycle is generally reduced. This is accomplished by dropping out from the life cycle some of the spore types. Such a life cycle is said to be microcyclic and the rusts are called microcyclic or short cycled rusts.
All the wheat rusts in India have been reported to be short cycle rusts. The haplophase which is ordinarily passed on the alternate host is altogether absent. There are no Barberry bushes or Mahonia plants in the plains.
The spermagonial or aecidial stages found on Barberry and Mahonia in the Indian hills have been found to have no connection with the wheat rust in the plains. The wheat rust in the plains, therefore, produces only two types of spores, the uredospores and the teleutospores.
Recurrence of Wheat Rust in the Plains of India. The black stem rust of wheat is a short cycled rust in India. The part of the life cycle involving the alternate host Barberry is cut out. It thus produces only uredospores and teleutospores.
The latter have lost power of germination. The uredospores cannot withstand the high temperature of summer months in the plains. There is thus no inoculum left in the fiels to infect the next wheat crop.
How does then the disease reoccur every year in the plains with equal vigour? Till recently Mehta’s hypothesis (1952, 1954) that the primary inoculum of black rust of wheat is introduced in the plains from the Himalayas in the North and Nilgiri and Pulney hills in the South held the sway.
According to Mehta’s hypothesis, at the higher altitudes (3,000-7,000 ft), the uredospores can over summer in the congenial temperature on self-sown plants, out of season crops, tillers and possibly on grasses.
The surviving uredospores serve as primary inoculum for the next wheat crop near or at the foothills. From there the infection spreads. The uredospores are blown by wind from the infected plants in the foothills to the healthy wheat plants in the plains.
Recent investigations of Joshi et al (1971-1974) and other workers do not support Mehta’s hypothesis so far as the role of Himalayas is involved.
They hold that the spring movement of black stem rust uredospores is from the South to North as is the general pattern of movement of black rust in most countries of Northern Hemisphere.
They argue that in the hills of Northern India, low temperature conditions prevailing during November to February are unfavourable for sporulation, infection, establishment and spread of the disease.
Rust pustules become inactive at low temperature (below 14°C). When the conditions become favourable in the month of March, there is very little time left for its spread as the wheat plants reach maturity.
In their view the principal source of infection of black stem rust of wheat in the plains of North is the dissemination of inoculum from the South and Central India.
The inoculum in the Himalayas is considered to be reintroduced from the plains in spring first in the foothills, then the lower elevations and finally at higher elevations.
Alternation of Generations in Puccinia Graminis:
The typical life cycle (Figs. 14.23 and 14.24) of Puccinia illustrates the important biological principle of alternation of generations. There are two distinct generations or phases in the life cycle.
One of these is the dikaryophase confined to the wheat plant. The other is the monokaryotic or haplophase passed on the alternate host (Barberry). The former is characterised by the binucleate condition of the cells.
It corresponds to the sporophytic phase. The dikaryophase in the life cycle is initiated by the germination of aecidiospores (aeciospores) on wheat and multiplied during the growing season of wheat by the formation of uredospores. Both are binucleate.
The dikaryotic mycelium first produces the binucleate uredospores (Fig. 14.24; 1 and 2) and later the binucleate teleutospores (Fig. 14.24; 4-6). Each cell of the young teleutospore has a pair of nuclei.
These however fuse (karyogamy) in the ripe teleutospore (14.24; 7). The cells of the mature teleutospore, each of which is equivalent to a probasidium, are the only diploid structures in the entire life history. They represent the reduced diplophase.
The young teleutospores are the last structures of the dikaryophase. The mature teleutospores represent the diplophase. The dikaryophase and the diplophase together constitute the sporophyte phase in the life cycle.
It starts with the aecidiospores and consists of the dikaryotic mycelium, uredospores and teleutospores.
Each diploid cell of the teleutospore germinates to produce the epibasidium (Fig. 14.24; 8). The diploid nucleus of each cell of the teleutospore (probasidium) migrates into its respective epibasidium and undergoes meiosis to form the haploid basidiospores.
This shows that karyogamy after disploidisation is considerably delayed but meiosis follows karyogamy immediately. Meiosis precedes the haplophase or the monokaryotic phase which is characterised by the uninucleate condition of the cells.
It starts with the basidia. The basidiospores (Fig. 14.24; 9) are the first haploid uninucleate spores in the life cycle. They germinate on the leaves of the alternate host barberry to produce the haplomycelium (Fig. 14.24; 10).
The latter produces the spermagonia and spermatia (Fig. 14.24; 12) and also the aecidial primordia. The aecidial primordia are the last structures of the haplophase.
The latter ends with the diploidisation of the basal cells in the aecidial primordia. With this the binucleate condition is re-established and dikaryophase again starts.
From the account given above it is evident that in the life cycle of Puccinia the dikaryophase and the diplophase constitute the sporophyte generation. It regularly alternates with the haplophase or gametophyte generation and vice versa.
One follows the other in a regular sequence. This means that the two generations constituting a single life cycle alternate with each other. In other words, Puccinia exhibits the phenomenon of alternation of generations.