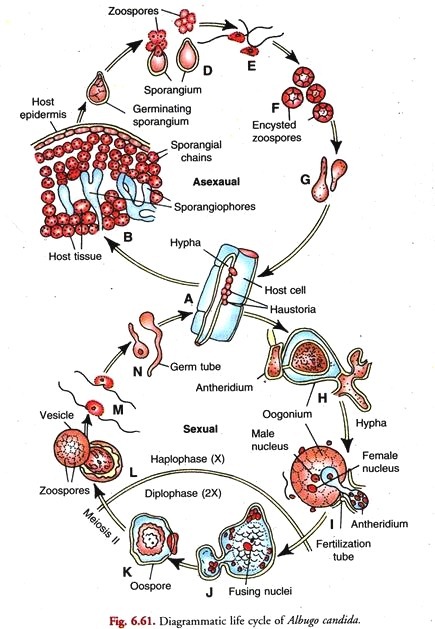
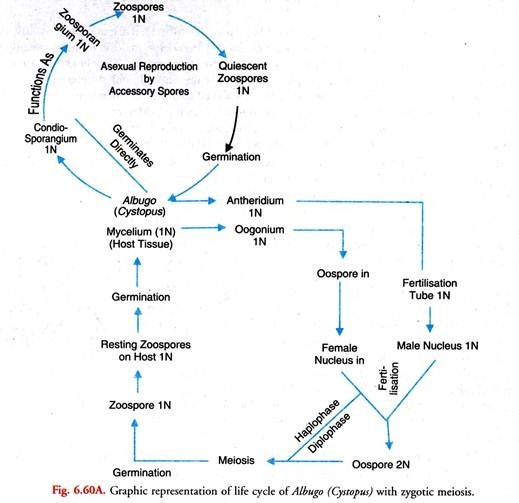
In this article we will discuss about the life cycle of albugo with the help of suitable diagrams.
Mycelium of Albugo:
It is well developed and consists of branched, aseptate, coenocytic hyphae. The hyphae live and ramify in the intercellular spaces of the susceptible host tissue. The hyphal wall contains cellulose and not chitin. The hyphal protoplasm is granular and vacuolate in the older parts.
It contains numerous nuclei, oil globules and glycogen. Electron micrographs reveal the presence of mitochondria, endoplasmic reticulum and ribosomes.
The cytoplasmic membrane which is closely appressed to the hyphal wall forms lomasomes. Septa remain suppressed in the actively growing hyphae but appear to separate reproductive structures and to seal off injured parts.
The fungus mycelium grows vigorously. The hyphae branch and ramify within the host attacking the tissues adjoining the point of infection. Sometimes both Albugo and Peronosporaoccur on the same host particularly Capsella bursapastoris. Albugo can, however, be distinguished from Peronospora by the smaller diameter of its hyphae and more numerous, vesicular haustoria (Fig. 6.50).
The intercellular hyphae of this obligate parasite produce intracellular haustoria in the mesophyll cells of the host (Fig. 6.50). The haustorium arises as a lateral outgrowth at the site where the hyphal wall is tightly pressed against the mesophyll cell wall.
An electron- dense amorphous material known as the penetration matrix is usually deposited at the site of contact between the host and the hypha cell walls. It is described as the penetration site (Fig. 6.51).
The slightly crescent-shaped bulge of the haustorial mother cell known as haustorial initial perforates the host cell wall at the penetration site and protrudes into the lumen of the mesophyll cell to develop into a haustorium. It is bordered by the invaginated host plasma membrane.
With light microscope the haustorium is seen as a small, spherical structure consisting of two parts namely:
(i) The haustorial stalk or neck and
(ii) The terminal haustorial head or body.
The haustorial stalk passes through the penetration site to connect the haustorial body to the hyphal wall in the intercellular space between the mesophyll cell. Usually one or two, sometimes more, haustona are seen in the thin peripheral layer of the host cell cytoplasm adjacent to the chloroplasts.
Reproduction in Albugo:
Asexual Reproduction in Albugo:
When the mycelium has reached a certain stage of maturity it epidermis produces pads of hyphae at certain areas just below the epidermis. The tips of hyphae constituting the mat grow verticlly into short, upright, thick-walled, unbranched club-shaped hyphae.
These are the sporangiophores (=conidiophores). They are arranged in a closely packed palisade like layer forming a sorus between the epidermis and the mesophyll of the host leaf. Each sporangiophore appears constricted at its junction with the subtending hypha.
The lower two-third portion of sporangiophore is narrow, thick-walled, with a undulating surface whereas the upper one-third is broader, thin-walled with a smoother surface. According to Khan (1977), the sporangiophore wall towards its proximal end consists of two layers, the outer more electron-dense and thicker than the inner layer.
(a) Abstriction of sporangia:
In the lower fungi (Phycomycetes) Albugo is unique in that its lemon- shaped sporangia are produced in basipetal chains at the tips of clavate sporangiophores. Two different views have been put forth to explain their mode of development.
According to one view, the sporangial chains in Albugo are abstricted by percurrent proliferation. The second view advocates the blastic mode of development.
(i) Percurrent Proliferation (Fig. 6.53):
The sporangia in Albugo which are cut off in succession are arranged in a basipetal chain on the sporangiophore. According to Hughes (1971), they are produced by successive proliferations of the sporangiophore subtending a sporangium.
This mode of development of sporangia is termed per-current proliferation. Generally the sporangiophore increases in length as each successive sporangium is cut off from each successive proliferation at a higher level than the previous one. The first formed sporangium is a aleuriosporangium.
Reaching a certain size it is delimited from the sporangiophore by a basal septum. The latter eventually splits into two halves so that the subsequent proliferation of sporangiophore involves the exposed half septum. In Albugo each successive sporangium is capable of seceding from the sporangiophore or from the young sporangium.
The second sporangium is thus formed by proliferation of the sporangiophore with total involvement of the half of the fractured transverse septum exposed by the seceding first sporangium above it. Apart from this, septum is seen at the apex of the young sporangium.
The second sporangium is delimited in the same manner as the first. As the second sporangium increases in size it pushes the first upward without disjunction. The process is repeated resulting in a chain of sporangia. Probably the septum seen at the apex of each younger sporangium thickens on both sides to form a connective between the successive sporangia in the chain.
According to Hughes, besides increase in length of sporangiophores, this method of sporangium development is accompanied by marked lamination and thickening of the walls of the sporangiophores. The mature sporangiophores are thus longer, more thick-walled and show annellations.
Thakur (1977) corroborated findings of Hughes (1971) on formation of sporangia by percurrent proliferation in Albugo.
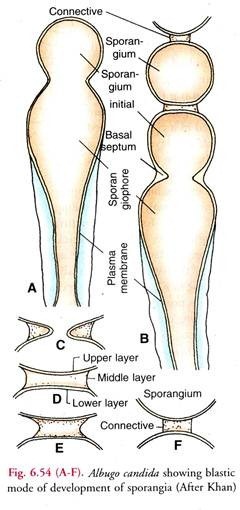
(ii) Blastic mode of Sporangium development (Fig. 6.54):
According to Khan (1977) the sporangiophore has a fixed sporogenous locus at its apex. The sporangial initial arises as a bud from it (A). It contains about 4-6 nuclei and dense cytoplasm.
The two wall layers of the sporangium initial are continuous with those of the sporangiophore wall. Reaching a certain size, the initial is delimited by a basal septum near the sporogenous locus. It becomes the first sporangium and the oldest in the chain (B).
The septum is formed by the centripetal growth of the inner layer of the sporangiophore wall (C). A nearly complete septum has a narrow central canal and consists of three layers, upper and lower electron dense and the thick middle one of less electron density (D).
After the completion of the basal septum and conversion of the initial into a full-fledged sporangium, a new sporangium initial grows as a bud from the sporogenous locus (B). It pushes the newly-formed sporangium upward. Thus only one sporangium is formed at a time.
As the second sporangium initial grows to the normal size, it is also delimited by the formation of a basal septum as the first. The repetition of the process results in the formation of a basipetal chain of sporangia. Soon after the formation of the first sporangium, the breakdown of its basal septum begains.
It is the middle layer which starts disintegrating from its periphery inwards whereas its upper layer fonns the wall of the upper sporangium and the lower layer completes that of the lower sporangium (E-F).
The fibrous product of dissolution of the middle layer is held in position by the pellicle which covers both the sporangia and the sporangiophore. It is seen as a connective or disjunctor between the successive sporangia in the chain. Khan (1977) did not notice any increase in length of the sporangiophore during sporangia formation nor did he observe any annellations on the sporangiophore surface.
Sporangia (Fig. 6.55 A, B):
They are small, hyaline, nearly spherical or lemon- shaped structures with a smooth or somewhat punctate surface.
The basipetal arrangement of sporangia in the chain (with the oldest at the top and the youngest at the base of the chain) serves two useful purposes:
(i) It permits ready dispersal of the oldest sporangia by air currents or rain water, and
(ii) It helps in the proper nourishment of the younger ones.
(i) Ultrastructure of sporangium (B):
According to Khan (1977), each newly formed sporangium is lemon-shaped and is about 19- 22 by 14-17 µ m in size. It bears remnants of the connectives or disjunctor pads at both the ends. The sporangium wall is differentiated into two distinct layers. The outer is more electron dense than the inner.
Within the sporangium wall is the highly convoluted plasma membrane enclosing the dense cytoplasm containing up to 4 nuclei. Besides, the cytoplasm contains endoplasmic reticulum, mitochondria, perinuclear, dictyosomes, ribosomes both free and attached to endoplasmic reticulum, vesicles of various kinds and lipid droplets.
Towards maturity the sporangial wall especially, its inner layer increases in thickness and the number of lipid droplets decrease as the sporangia matured. The oldest sporangia have none.
The endoplasmic reticulum becomes accumulated in the peripheral cytoplasm. Towards the end of sporangial maturation, the dictyosomes become quiescent, mitochondria decreased in number and also the amount of endoplasmic reticulum. The sporangial wall increased 3-fold the thickness.
(ii) Dispersal of sporangia:
The chains of sporangia lengthen and press on the epidermis above. This causes the leaf surface to bulge. The overlying epidermis eventually bursts over the growing sporangial sorus and exposes the white shining pustules consisting of masses of sporangia.
The pustules look like white blisters. The exposed sporangia are white. The distal ones by this time have matured. As the sporangia mature the connectives or gelatinous pads between them dry, shrink and finally disintegrate in moist air. The sporangia in the chain thus separate. They are then blown away in the air by wind or washed away by rain water.
Germination of sporangia (Fig. 6.56):
Landing on a suitable host the sporangia begin to germinate within two or three hours under suitable conditions.
At the time of germination they behave in either of the following two ways depending on temperature conditions:—
Indirect Germination (Fig. 6.56 B-E):
In the presence of moisture and low temperature, the sporangium functions as a zoosporangium (B). The optimum temperature for germination of sporangia is 10°C. It absorbs water and swells. A few vacuoles appear in its granular cytoplasm.
Later the vacuoles disappear and the multinucleate protoplast undergo division. It divides to form five or eight polyhedral uninucleate daugher protoplasts. Meanwhile an obtuse papilla forms on one side of the sporangium.
Each daughter protoplast shapes into a slightly concave-convex zoospore (E). It has a disc-like contractile vacuole on one side and is furnished with two flagella, one short and one long. The former is of tinsel type and the later whiplash. The flagella are attached laterally near the vacuole.
As the zoospores are differentiated, the papilla swells and opens. The zoospores still immobile, emerge usually one by one (Fig. 6.56 C). According to Vanterpool, the zoospores are, at first, released in a sessile vesicle formed by the swelling of the papilla. The vesicle soon vanishes.
Germination of zoospore and infection of host (Fig. 6.56 F-H):
Moisture on the surface of the host is essential for germination and infection. The released zoopores swim about in water for a while (E). Finally they settle down on the host, retract the flagella and round off.
Each secretes a wall around it (F). The encysted zoospore (cyst) then germinates. It puts out a germ tube (G) which gains entrance into the host thought a stoma (H). Once within the host tissue the germ tube grows and forms the mycelium
(ii) Direct Germination (Fig. 6.56 I-J):
At high temperature and under comparatively dry conditions the sporangium behaves like a conidium (I). It germinates directly to form a germ tube (J). The conidial method of germination of sporangia in Albugo is, however, not common.
The germ tube penetrates the host through a stoma or, through an injury in the epidermis. Within the host it develops into a mycelium. Re-infection of the host and infection of other healthy plants in the vicinity goes on by the production of sporangia throughout the growing season.
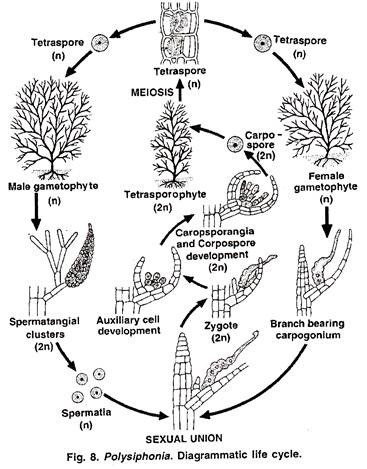
Sexual Reproduction in Albugo:
It is oogamous:
The male sex organ is called an antheridium and the female oogonium (A). They are developed near each other in the intercellular spaces of host tissues towards the end of the growing season. When the mycelium ages, some hyphae grow deep and lie buried in the intercellular spaces of the tissues of the stem, or petioles.
The sex organs arise on separate hyphae called the male and the female hyphae (A). The two soon establish contact. The antheridium comes in contact with the oogonium at the side. The development of sex organs within the host tissue is externally indicated by hypertrophy and distoration in shape in the particular organ.
(a) Oogonium:
It arises as a globular enlargement of the tip of the female hypha. Sometimes the oogonium is intercalary in position. The swelling is multinucleate(A) Across wall appears below this inflation (B). It separates the terminal oogonium from the rest of the female hypha. The young ooglum has highly vacuolated contents.
The numerous nuclei and vacuoles are evenly distributed and the usual cell organelles are dispersed throughout the oogonium. The nuclei divided mitotically and increase in number as the oogonium advanced towards maturity. After the first Sc division the ooginial cystoplasm shows marked zonation (C).
It becomes differentia into two distinct regions with the rearrangement of the numerous nuclei and other cellular organellers. Most of the original cytoplasm of the oogonium forms the central,rounded dense ooplasm. It is multinucleate and contains only a few mitochondria,ribosomes and cisternae of ER. It is rich in lipid vesicles and reserve vesicles containing electron dense inclusions (reserve globules).
The ooplasm is surrounded by the peripheral cytoplasm constituting the periplasm. It is more vacuolate and spongy. The vacuoles are large. Besides, the periplasm is rich in nuclei, mitochondria endoplasmic reticulum and ribosomes. It has protoplasm of thinner consistency. Sometime after all the nuclei of ooplasm migrate into the periplasm (D) and become arranged in a ring.
Here they divide mitotically with the spindles lying in such a way that one pole of each that one pole mitosis one daughter nucleusthe ooplasm and the other in the periplasm (E). At the end of mitosis one daughter nucleus of each spindle goes to the ooplasm and the other to the periplasm (F). However, the ooplasm at maturity has a single centrally located nucleus (G).
There are two views with regard to this uninucleate condition of the mature ooplasm. According to one view, all the nuclei excepting one are extrude from the ooplasm and are deposited in the periplasm. The second view IS that in the later stages of development all the nuclei in the ooplasm excepting one degenerate and disappear. The uninucleate ooplasm functions as the female gamete or egg or oosphere (G).
(b) Antheridium:
It is an elongated club-shaped cell (A). It is multinucleate. The antheridium is developed at the end of a male hypha lying close to the oogonium. The end of the male hypha enlarges into club-shaped swelling.
The latter is then cut off by a cross wall from the rest of the male hypha (B). This terminal dub-shaped cell is called an antheridium. It contains several nuclei (usually 6-12), but only one is functional. The paragynous antheridium comes in direct contact with the oogonium at the side (C).
(c) Fertilisation:
At the point of contact of antheridium with the oogonium, the walls become very thin. A portion of the contents of the oogonium surrounded by a thin membrane grows into a papilla-like outgrowth (G).
This papilla-like oogonial bulging is called the receptive papilla It is functionless. The receptive papilla bulges (G) into the antheridium but soon disappears. This is followed by the formation of a slender tubular outgrowth from the antheridium.
It is the fertilisation tube (H). The fertilisation tube passes through the thin spot in the oogonial wall and enters the multinucleate periplasm. It then dips deep into the ooplasm. Prior to this a spherical and granular cytoplasmic body appears in the centre of the oosphere (H). It is known as the coenocentrum. The single functional female nucleus is attracted towards it and becomes attached to a point near it.
The fertilisation tube finally reaches the coenocentrum and ruptures (I) at the tip to introduce a single male nucleus which fuses with the female nucleus. Thereafter the fertilisation tube collapes but persists and the coenocentrum vanishes. On removing or displacing the attached antheridium Tewan and Skoropad (1977) observed a clear hole surrounded by some fibrous material.
Oospore (Fig. 6.58 A-C):
Tewari and Skoropad (1977) investigated the fine structure and development of A. Candida oospores. According to them, the young oospore is delimited from the vacuolate periplasm by an electron-dense cell wall.
The single layered cell wall of the young oospore encloses dense cytoplasm containing a group of reserve vesicles, lipid vesicles and a few membranous organelles. It is surrounded by periplasm rich in vacuolate cytoplasm containing membranous organelles.
Further development of oospore if marked by the deposition of 4 layers, two on the outer and two on the inner side of the first (original) layer of the young oospore. The mature oospore thus has a thick highly differentiated 5-layered wall.
External to the oospore wall are the two addition protective investments formed by the persistent periplasm and the oogonial wall. The thick highly differentiated oospore wall together with the two surrounding additional layers contributes to the longevity of Albugo oospore. The authors suggest that periplasm plays an active part in deposition of oospore wall layers.
Within the fully developed oospore wall is the scanty cytoplasm surrounding a large central reserve globule. Some small bodies resembling the reserve globules in appearance and numerous lipid vesicles occupy most of the space between the oospore wall and the central reserve globule.
The highly differentiated thick, oospore wall together with the two additional layers constituted by the persistent perisperm and the oogonial wall provides protection and the numerous lipid vesicles in the oospore cytoplasm furnish energy for the long dormancy or overwintering by oospores in Albugo.
The outer layer of the oospore wall is comparatively thicker. It is warty or tuberculate. In other species it may have a network of ridges or other patterns.
Location of meiosis:
The place of meiosis in Albugo is still under dispute. Stevens (1899) suggested that Albugo possesses diploid somatic nuclei which undergo meiosis in the gametangia (antheridia and oogonia). His interpretation was disputed by later workers. They held that Albugo and other Oomycetes, as a whole, are haploid. Oospore is the only diploid structure in the life cycle.
It undergoes zygotic meiosis. Walker (1969) observed that after fertilization when a thick wall is being developed around the oospore, its diploid nucleus divides repeatedly to form 32 nuclei. Probably the two earlier of these divisions constitute meiosis. In this 32 nucleate stage, the oospore enter the resting stage and tides over the period unfavourable for growth.
However, Sansome and Sansome (1974) have advanced evidence in support of gametangial meiosis and diploid life cycle in Albugo Candida. They hold that the first two divisions of nuclei which occur in the gametangia constitute meiosis. This view is gaining ground.
Germination of Oospore (Fig. 6.59):
On the onset of conditions favourable for growth, the oospore germinates. The central globule and the lipid droplets gradually disappear. The contents of the oospore assume uniform granular appearance.
The diploid nucleus undergoes repeated divisions to form many nuclei (about 100 or even more). A small amount of cytoplasm gathers around each daughter nucleus. Numerous uninucleate daughter protoplasts thus result. Each of these metamorhoses into a biflagellate zoosore.
The oospore then germinates to release the zoospores by either of the two following methods:—
(i) The thick oospore wall cracks. A germ tube emerges through the split. It ends in a thin vesicle. From the oospore the zoospores pass into the vesicle. Soon the vesicle perishes to liberate the zoospores.
(ii) Through a crack in the oospore wall emerges a thin sessile’ vesicle containing the zoospores (A). The vesicle soon bursts to liberate the zoospores.
(iii) Verma and Petrie (1975) described direct germination of oospore without the intervention of zoospores. In this method after the disappearance of the central globule and the lipid dropletes the contents of oospore assume uniform granular apearance.
The thick oospore wall then cracks. The contents then emerge in the form of one or two simple or branched germ tubes. Direct penetration of the host by a germ tube has not been reported. Good germination occured at 10-20°C. They observed that 71 percent of the oospores collected from the field in August germinated within two weeks.
Structure of zoospore:
The liberated zoospore is a biflagellate, uninucleate structure which is reniform in shape. The two unequal flagella arise from a depression on the concave side. The shorter flagellum is of tinsel type and the longer one of whiplash type. The former is directed forward and the latter trails behind when the zoospore is in motion.
Germination of Zoospore (Fig. 6.59 D-E):
On coming in contact with a suitable host. The flagella are withdrawn. It then rounds off and secretes a wall around it (D). Soon the encysted zoospore (cyst) puts out a germ tube (E) which enters the host tissue through a stoma. Once within the host tissue the germ tube grows vigorously and forms a new mycelium.
Primary infection of host:
According to Verna et al. (1975), the zoospores produced in the germinating oospores serve as a primary inoculum for infection of the susceptible host. The emerging cotyledons are the infection sites.
After penetration the first haustorium originates near the tip of the young hypha. The latter then continues to grow leaving the haustorium as a side branch. The formation of the first functional haustorium is the critical step in primary infection. It indicates the establishment of a compatible functional host-parasite relationship.
From then onwards hyphal growth increases rapidly. The hyphae grow around the palisade mesophyll cells with haustoria penetrating the adjacent cells. The number of haustoria per cell varies from one to several. In the course of time a mycelial base is established inside the host tissue (cotyledons).
It produces masses of zoosporangia on the cotyledons which serve as secondary inoculum in initiating systemic infection. The parasite ultimately reaches the inflorescence region where it produces the oospores.