In this article we will discuss about the development of basidiomycetes, explained with the help of suitable diagrams.
In a large number of Basidiomycetes the dikaryotic somatic mycelia, both secondary and tertiary intertwine producing highly organized sporocarps or fruiting bodies or fructifications or sporophores which are known as basidiocarps. Whereas, in a relatively few Basidiomycetes there is total absence of the development of basidiocarps.
The basidiocarps may be epigeous or hypogeous, or there may be many transitional forms between these conditions.
They are extremely variable in size, texture, colour and shape ranging from very large macroscopic to even minute microscopic in size. The basidiocarps besides being thin crust-like cartilaginous, or fleshy, spongy, gelatinous, corky, woody, may be almost of any other texture. So also in colour they may be bright orange-red, yellow, brown, dark, white and different grades of these and some other shades of colour.
The basidiocarps are often so highly developed and attract attention so easily that the main body of the fungus which is the extensive mycelium, usually goes unnoticed. Most common ones are crust-like, partly or completely shelved without any stalk; or stalked umbrella-like, fan-shaped, coralloid, round to oval and of various other configurations.
Structurally the basidiocarps may also vary enormously. But In general they are composed of fertile tissue, the hymenium or the hymenial layer produced by the secondary mycelium and sterile tissue, the trama or context (Fig. 253G & E) of tertiary mycelium. The hymenial layer may be smooth or spread along plate-like structures known as gills or lamellae.
The part of the basidiocarp bearing the hymenium is known as hymenophore. The basidiocarp is often covered by a covering known as peridium (Fig. 253H).
The Basidiocarp may be:
(i) Open without being covered by a peridium from the very beginning of its development exposing the hymenium— gymnocarpous.
(ii) The hymenium is enclosed by basidiocarp tissue at first but later becomes exposed to the open air before the spores are mature—hemiangiocarpous.
(iii) The fertile portion (gleba) of the basidiocarp is enclosed in basidiocarp tissue until well after the spores are matured—angiocarpous, and
(iv) When the mature hymenium remains covered—endocarpous (Fig. 253G & H).
The hymenium may be borne on all sides of the basidiocarp—amphigenous, or on one side only—unilateral. In cases where the basidiocarps are closed or closed up to maturity of spores, enclosed within the peridium are the tramal plates lined with hymenium, known as gleba (Fig. 253H). The tramal plates and the hymenium are ultimately transformed into a characteristic mass, it is called glebal mass.
Where the basidiocarps are open from the very beginning of their development, the spores are dispersed mostly by a special mechanism known as fluid drop or drop-excretion mechanism of dispersal of spores. Whereas, in basidiocarps opening at a later stage, the spores are dispersed by agencies like wind, raindrops, animals and insects.
Again in others, where the basidiocarps remain closed, the spores are liberated by the disintegration or accidental breaking of the basidiocarps.
The hymenium of a basidiocarp consists of basidia and sterile structures interspersed with them. These sterile structures are often regarded as immature basidia. But they are generally designated as paraphyses (sing, paraphysis) (Fig. 253E) and have been compared with those of the Ascomycetes. This has been questioned by Leutz (1954) and others.
According to them, the sterile structures of the hymenium are nothing but the sterile hyphal ends which are basically, just like basidia, nothing but the components of the dikaryotic secondary mycelium.
Whereas, the asci and paraphyses in the Ascomycetes originate from different hyphal elements. As such the similarities between the paraphyses of the Basidiomycetes and the Ascomycetes are superficial and they are not homologous structures. Hedwig (1789) was the first person to use the term ‘paraphysis’ in relation to fungi. Functionally the paraphyses are considered as spacing organs.
Besides paraphyses, the hymenium may also have certain other sterile structures whose taxonomic interest has been emphasized largely by Bourdot and Galzin (1928), and Overholts (1929).
Some of them are:
i. Acanthophyses (sing, acanthophysis):
These are sterile hyemenial hyphal ends having a number of short pin-like outgrowths on their surface. Burt (1914) designated them as ‘bottle-brush paraphyses’, present in certain species of Aleurodiscus (Fig. 254F).
ii. Dendrophyses (sing, dendrophysis):
These are hyphal ends of the hymenium or context having irregular tree-like branching. They are thick- or thin-walled and covered throughout with spines of variable length, also encountered in certain species of Aleurodiscus (Fig. 254C).
iii. Dichophyses (sing, dichophysis):
These sterile hyphal ends arising from the hymenium or subhymenium are antler-like in appearance because of their successively dichotomous, wide-angled branching and the prong-like terminal branchlets (Fig. 255A). They are present in some tropical species of Hymenochaete and species of Vararia.
iv. Pseudophyses (sing, pseudophysis):
These are smooth thin-walled, unbranched hymenial structures which are knobbed, knotted or nodose or moniliform (Figs. 254F and 255B). They are present in species of Aleurodiscus and Coprinus.
v. Gystidia (sing, cystidium):
These are hymenial or subhymenial organs, often projecting beyond basidia. They are elongated subcylindrical globose to sub- globose (Fig. 254A & B); hyaline to uniformly coloured or wall coloured and sap not coloured, never dark-brown or black even in KOH. They are present in species of Stereum, Pork, Armillariella, Coprinus, Lentinus, etc.
The cystidium has more or less rounded tip which may be smooth or provided with spines. The wall of the cystidium may have deposition of Ca-oxalate crystals, found in species of Peniophora.
The existence of cystidia was first demonstrated by Micheli (1729) and was put to wider use by Hedwig (1789). The taxonomic importance of cystidia in the study of the family Thelephoraceae has been introduced by Massee (1889) and later on adopted by Burt (1914-1926) and many others. In some fungi, e.g., Vararia sp., cystidioid prolongations occur in the hymenium.
These structures do not possess staining qualities of true cystidia and are known as pseudocystidia (sing, pseudocystidium) (Fig. 255A).
vi. Gloeocystidia (sing, gloeocystidium):
These are subhymenial organs extending up to the hyemnium, may or may not remain associated with cystidia. They are of varied form, some of them being nearly indistinguishable from submerged, non-incrusted cystidia, others being long, flexuous, refractile structures.
A typical gloeocystidium is an elongated, usually rather slender, tortuous object, sometimes slightly enlarged toward the base and with a long slender apical neck, sometimes slender over the entire length and then often tapering very gradually toward the apex (Fig. 254G).
The walls are thin and colourless, very rarely with true septa. The walls of gloeocystidia are never incrusted with crystalline particles. The gloeocystidium contains sap which on mounting in glycerine divides into several opaque blocks, orange or brown in colour containing oily substances which do not exude when the gloeocystidium is broken.
The gloeocystidia are especially prominent in the genera Aleurodiscus, Stereum, and Merulius.
vii. Setae (sing, seta):
These are hymenial and subhymenial organs, mostly subhymenial and extended up to hymenium. The setae may be broadly ovate in shape and may extend beyond hymenial level. They are usually bristle or bristle-shaped bodies, typically deep yellow or brown in colour and dark-brown or black when treated with KOH. Setae contain brown-coloured sap.
Their tips may be pointed (Fig. 255G), blunt, curved or twisted. The genus Hymenochaete is characterized by having setae. Setae differ from most cystidia by their brown colour. When the setae have several radiating spines they are known as stellate setae. A stalked stellate seta is designated as asterophysis.
viii. Hyphal pegs:
Compound hyphal fasciculate projections derived from undifferentiated hyphae extend beyond the general level of the hymenium forming the hyphal pegs. Each hyphal peg consists of two or more parallel or interwoven hyphae forming a soft column (Fig. 254D). It may be pyramidal or cylindrical or conical in outline.
The hyphal pegs are found in the members of the families Thelephoraceae, Polyporaceae and Agaricaceae.
ix. Latex vessels:
These are elongated, branched, non-septate, labyrinthiform, anastomosing hyphae of variable thickness. They rise from the subhymenium, penetrate the hymenium growing between the basidia and end on the upper surface of the hymenium (Fig. 254E). The latex vessels are multinucleate, vacuolate structures which contain milky or coloured or a hyaline sap which becomes coloured upon contact with air. They are present in Corticium seriate.
Development of Basidium and Basidiospores:
A basidium develops from a dikaryotic terminal cell of a hypha of the secondary mycelium. The dikaryotic terminal cell is separated from the rest of the hypha by a septum over which a clamp connection is usually found. During the development of the basidium the terminal cell enlarges to form young basidium, also known as basidiole (Fig. 256A to D).
The two nuclei of the basidiole fuse to form a diploid nucleus (Fig. 256B & G). The diploid nucleus then undergoes meiosis producing four haploid nuclei. The growing basidium then produces four protuberances at the top. These protuberances are known as sterigmata (sing, sterigma) (Fig. 256D).
They may be lacking in some Basidiomycetes. When present, the tips of the sterigmata enlarge to produce basidiospore initials. The four haploid nuclei then squeeze through sterigmatal passage into each basidiospore initial. The basidiospore initial then becomes walled off to form a basidiospore with a single nucleus (Fig. 256E).
The portion of the basidiospore in contact with the sterigma has been designated by Heim (1931) as hilum; and hilar appendix or apiculus to the short, often sharp protrusion near the hilum at the basal end of the basidiospore.
Each basidium typically bears four basidiospores on four sterigmata, a tetrasterigmate basidium (Fig. 256E). The basidiospores may be borne symmetrically or asymmetrically on the sterigmata. Sometimes a basidium may produce only two sterigmata and two basidiospores. Such a basidium is called bisterigmate basidium (Fig. 257G).
In such case each of the two spores may receive two nuclei resulting in the development of binucleate basidiospores. In cases of bisterigmatic basidia the basidiospores may also be uninucleate. Here, the other two nuclei remain unused within the basidium and eventually disintegrate. The number of basidiospores may also be more than four.
The basidia are usually slender, clavate to broadly clavate. Spherical to elongate basidia are also not uncommon. They may be septate or aseptate. Septation is usually transverse or vertical, may also be oblique. During nuclear division the orientation of the nuclear spindles may also vary in different basidia.
When the nuclear spindles are oriented transversely to the basidium, such basidia are regarded as of the chiastobasidial type. Again basidia having nuclear spindles oriented longitudinally or obliquely are the stichobasidial type.
Following are the main types of basidia:
i. Homobasidia:
The basidia are slender, clavate to broadly clavate, or nearly globose and aseptate. The number of basidiospores may be four or more, with (Fig. 257A, B, F & H) or without sterigmata (Fig. 257E & G). They are also known as autobasidia or holobasidia. The homobasidia may be chiasto-, or stichobasidial type.
When the basidia are developed inside a fruit body, they are the endobasidia. Again basidia with basidiospores symmetrical on sterigmata are the apobasidia and those with basidiospores asymmetrical are the autobasidia.
ii. Heterobasidia:
The basidia are either septate or deeply lobed. They are also known as phragmobasidia or metabasidia.
In heterobasidia, the enlarged basal portion of the mature basidium in which nuclear fusion takes place has been designated by Neuhoff (1924) as hypobasidium and which bears upon it the epibasidium, but not the sterigmata directly. According to Neuhoff the hypobasidium and the epibasidium make up a heterobasidium.
Linder (1940) discarded this terminology and preferred probasidium for hypobasidium and basidium for epibasidium.
The Heterobasidia may again be:
(i) Transversely septate.
(ii) Longitudinally septate, and
(iii) Deeply lobed.
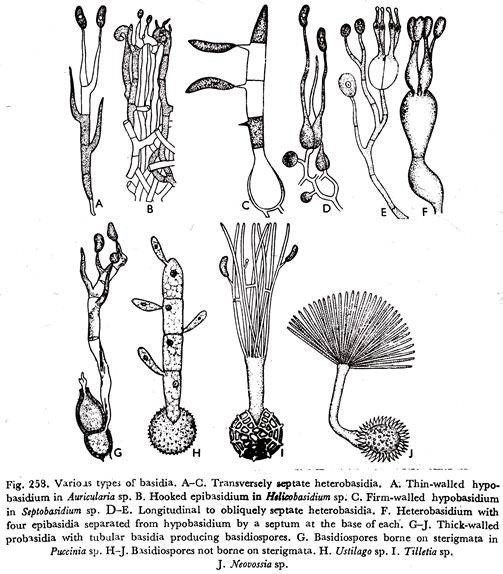
The transversely Septate Basidia may be differentiated into:
(a) With thin-walled hypobasidium and the epibasidium is traversed by three septa. Each cell of the epibasidium produces a long tube at whose apex a sterigmatic structure is formed on which arises the one-celled uninucleate basidiospore. (Fig. 258A). The basidiospores are at the same level, as in the genus Auricularia.
(ii) The hypobasidium produces hooked epibasidium which again produces rather long sterigmata (Fig. 258B), seen in the genus Helicobasidium.
(ii) The hypobasidium is firm-walled from which grows out the straight or curved epibasidium which becomes four-celled. The basidiospores are borne on well-developed sterigmata (Fig. 258G), found in the genus Septobasidium.
(d) In the Uredinales and Ustilaginales the mature teleutospore, a uninucleate but diploid thick-walled structure, is the probasidium. On germination it gives rise to a tubular basidium, designated as a promycelium. By meiotic division of the diploid nucleus and by transverse septation, the promycelium becomes four-celled.
Each cell of the promycelium contains a single haploid nucleus which takes part in the development of a basidiospore. In the Uredinales the basidiospores are borne on sterigmata and number of basidiospores in four (Fig. 258G). Whereas, in the Ustilaginales there is absence of sterigmata and more than four basidiospores are formed (Fig. 258 H to J).
The longitudinally septate basidia are the so-called cruciate type. The hypobasidium is longitudinally septate. The nuclear spindle is chiastobasidially oriented.
Two vertical or oblique septa are formed at right angles to one another producing a four-celled structure from each of which arises a prolongation—the epibasidium which is terminated by a sterigma, bearing the basidiospore (Fig. 258 D & E). The various genera of the Tremellaceae exhibit this form a basidial character.
In the members of the Tulasnellaceae the basidium is not divided by vertical septa. The hypobasidium is subglobose, pyriform or broadly clavate. From the upper portion of the hypobasidium arise usually four stout cells—the epibasidia, each of which is narrowed at the tip to form a sterigma upon which a single basidiospore is formed. The epibasidia are separated from the hypobasidium by a septum at the base of each (Fig. 258F).
In the Dacrymycetaceae the basidia, when young, are long cylindrical or somewhat clavate. The basidial nucleus divides stichobasidially. The broadened apex of the basidium becomes deeply lobed to produce two epibasidia and almost of the same length as the hypobasidium. Each epibasidium is terminated by a sterigma at whose apex the basidiospore is produced.
The basidium takes a ‘tuning fork’ in appearance, from which it is known as tuning fork type of basidium (Figs. 257G & D and 277 G to J).
The Basidiospores:
The basidiospore is a haploid structure. Basidiospores are generally unicellular, uninucleate and thin-walled structures with the exception of some septate and some thick-walled ones. They may be globose oval, elongated, or sausage-shaped or apically truncated with cut top. Their colour en masse, may be hyaline, pink yellow, green, orange, brown, or black.
The basidiospore wall is almost universally smooth, except a few cases where wall markings are present. Some basidiospores give a positive amyloid reaction by being coloured blue with Melzer’s reagent. Again some thick-walled spores absorb aniline blue dye strongly to the, inner-wall surface.
Such spores are said to be cyanophilous. Basidiospores are asterigmate (Fig. 257E & G) when the sterigmata are lacking. But usually they are sterigmate (Fig. 257A & B), i.e., borne on sterigmata. The sterigmate basidiospores may be symmetrical or asymmetrical depending on whether they are arranged symmetrically or asymmetrically on the sterigmata. In majority of the Basidiomycetes, the basidiospores are arranged asymmetrically on the sterigmata.
In most of the Basidiomycetes where the basidiospores are produced in the open air there is usually a special provision for the discharge of these spores from their points of attachment. The spores are usually discharged with considerable violence. The term ballistospore has been applied to violently projected spores (Derx, 1948).
Whilst most basidiospores are ballistospores, some, e.g., those of the Gasteromycetes, are not. Basidiospores on a four-spored basidium are discharged in a regular succession and not simultaneously. The interval between discharge of the first and second spore may be a minute or more; that between discharge of the second and third, or the third and fourth spore may be somewhat longer.
Since the maturity of basidia is usually a prolonged process, the period of basidiospore discharge is also prolonged which may last for hours, days or even weeks. The present-day knowledge of spore discharge among the Basidiomycetes is from the researches of Buller (1909-1933).
Buller observed that in cases where the basidiospores are attached asymmetrically on the sterigmata, the spores are discharged violently by the mechanism which is termed the drop-excretion mechanism, also known as fluid drop mechanism (Fig. 259A).
A minute or so before the spore discharge, a small droplet of liquid exudes at the hilum (apiculus) (Fig. 259B to D), it is often referred to as ‘Buller’s drop’. Within 5 to 20 seconds this liquid drop increases in volume to about one-fifth the size of the spore and then both spore and liquid drop suddenly shot off from the sterigma (Fig. 259E). The sterigma may or may not collapse after the spore is discharged.
Buller (1909) initially postulated that basidiospore discharge was due to the sudden rounding-off of the contacting walls of the spore and sterigma. He subsequently (1922) rejected this hypothesis and suggested that a pressure developing in the vicinity of the hilum was the source of the force for discharge. Buller also suggested that the force for discharge is caused by surface tension energy.
Ingold (1939) explained that by the time the liquid drop flow is at the hilum, the spore also will tend to move in the opposite direction exerting pressure on the end of the sterigma. This pressure causes the discharge of spore. Corner (1948) stated that the liquid drop formed at the hilar appendix was surrounded by a membrane, which, he suggested, was an extension of the wall of the sterigma.
Olive (1964) suggested that the discharge was due to the explosion of a gas bubble in the vicinity of the hilar appendix and to the residual gas between the inner wall and the outer membrane of the spore and sterigma.
Wells (1965) with the electron microscope studied the ultras structure of the basidia and basidiospores in Schizophyllum commune. He observed that a thin fragile extension of the wall of the sterigma is present around the base of the basidiospore. The liquid drop is formed within that portion of the extension of the sterigma wall covering the hilar appendix.
The enlargement of the drop contributes to the rupturing of the contacting surfaces between the sterigma and the spore, and to the force of discharge. Besides this, the turgour pressure within the basidium also contributes to the force of basidiospore discharge.
The basidiospores germinate in presence of moisture by germ tubes and produce primary mycelium. The uninucleate basidiospores produce monokaryotic primary mycelium. But in case of binucleate basidiospores the condition is different. A basidiospore may be uninucleate in origin but subsequently become binucleate by the mitotic division of the single nucleus.
Such a binucleate basidiospore behaves like a uninucleate basidiospore. Whereas, it may so happen, two of the four haploid nuclei of the basidium may pass into a single basidiospore and the other two in another basidiospore. Here the binucleate basidiospores possess two nuclei which are genetically different. Such binucleate basidiospores give rise to dikaryotic secondary mycelium.
Again basidiospores instead of germinating by germ tubes may bud out large number of conidia from which the mycelium is produced.
In the Basidiomycetes there are both homothallic and heterothallic species. Since the term heterothallic as originally applied refers to mycelia representing different sexes, the use of the terms heterothallic and homothallic in the Basidiomycetes should be avoided, if not otherwise qualified.
It is advisable to use self-compatible or self-fertile and self-incompatible or interfertile for homothallic and heterothallic respectively. The large majority of the Basidiomycetes are self-incompatible and rather few are self-compatible.
Among the self-incompatible Basidiomycetes, the compatibility may be governed by one allelomorphic pair of factors, Aa, located on different chromosomes, or by two pairs of allelomorphic factors Aa Bb located on different chromosomes and segregating independently.
The former condition is known as bipolarity, the corresponding species bipolar species; and the latter is designated as tetrapolarity, the corresponding species tetrapolar species.
The common explanation of these phenomena is that in bipolar species fruit body development is dependent on two factors and in tetrapolar species on four. Basidium of a tetrapolar species again will produce two kinds or four kinds of basidiospores depending on the method of chromosome segregation during meiosis and on crossing-over.
When segregation of the factors controlling compatibility takes place in the first division, only two kinds of nuclei will result; AB, AB and ab, ab or Ab, Ab and aB, aB.
In such case when mycelium bearing nuclei containing AB or Ab unites with mycelium having nuclei possessing ab or aB, a dikaryotic condition, bearing all factors Aa Bb in each cell of the dikaryotic mycelium will be established to produce basidia and basidiospores.
If, however, segregation takes place at the second division of meiosis, a basidium will give rise to four types of spore: AB, Ab, aB, ab. Basidia and basidiospores are produced only when the secondary mycelium has the combination of compatibility factors of Aa, Bb.