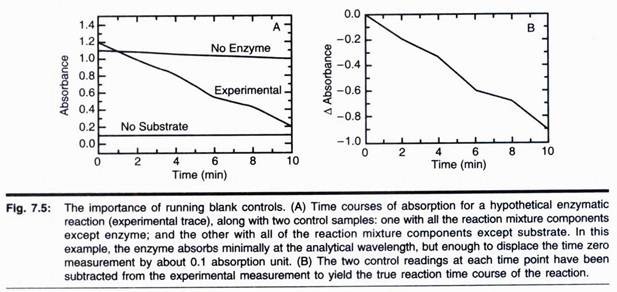
In this article we will discuss about the phylogenetic relationships of phycomycetes.
Fascinatingly speculative accounts have appeared from time to time with regard to the origin and phylogeny of the Phycomycetes without leading to any agreement. More critical and comprehensive work will have to be taken up on the various groups of lower organisms before drawing any widely acceptable conclusion.
Widely diversified views have been put up by various workers which may be indicated in the following lines:
1. The Phycomycetes are degenerate algae. The statement was made only taking into consideration the superficial resemblances between the two groups Algae and Fungi, but completely overlooking the fact that their physiological processes are quite different.
The Algae and Fungi probably arose as separate groups from unicellular chlorophyll-bearing and non-chlorophyll-bearing organisms respectively.
2. The two groups have evolved as two parallel series was suggested by De Bary and his student Vuillemin 1912 and was given prominence by Atkinson 1909, 1915 and later by Fitzpatrick. In a number of points of morphology and behaviour, the Fungi differ from Algae, e.g., zoospores, structure and mode of sexual reproduction, etc.
The fungi have no connection with the algal line and are derived from primitive colourless organisms below the level of the Chytridiales.
3. The origin of the Phycomycetes is to be sought among non-pigmented flagellates with which there are many points of likeness. It is probable that the Fungi have evolved from lower forms along lines independent of those leading to green plants and to animals.
4. The Phycomycetes have a protozoal ancestry and should be placed in a position distinct from the Algae.
5. The aquatic Phycomycetes were considered by A. Scherffel 1925 to be made up of two distinct series, the Chytridineen and the Saprolegnineen-Peronosporineen. These he believed to have arisen from different monad ancestors, the first from an uniflagellate type, and the second from a biflagellate, and to have undergone independent development. Scherffel’s idea was supported by Sparrow.
Sparrow (1958), however, was of opinion that the Phycomycetes are not a homogeneous monophyletic group, but rather an artificial category of coenocytic zoos- poric fungi, consisting of four groups which may have come from radically different progenitors.
The evolution of the lower fungi has become of current interest through Koch’s (1956) discovery of the presence of what he called a second, non-functional, vestigial blepharoplast in the chytrid motile cell, suggesting a biflaggellate ancestry.
This supposition was supported by conclusions from observations on the movement of chytrid zoospores. Koch observed, that the double image of the flagellum formed when the flagellum is undulating in a plane horizontal to the observer, may be asymmetrical, with one side more ‘humped’ than the other.
He suggested that this asymmetrical double image originates phylogenetically in a motile cell in which the posteriorly directed flagellum is laterally inserted on the body, so that the primitive or ancestral condition is not the posteriorly attached whiplash flagellum, but rather the laterally attached whiplash flagellum.
This, and the second non-functional blepharoplast suggest that the chytrids, and perhaps all posteriorly uniflagellated fungi, have evolved either from biflagellated organisms or from organisms with biflagellated swimming cells.
Order Chytridiales:
The members of this order, commonly known as chytrids, are parasites or saprophytes usually in water, rarely on land. They are regarded as the lowest of all the fungi. Some of the chytrids induce serious diseases of plants of economic importance, for example,
(i) Synchytrium endobioticum causes wart disease of potato
(ii) Physoderma Zeae-maydis causes brown spot of maize
(iii) Urophlyctis alfalfae causes crown wart of alfalfa
(iv) Olpidium brassicae causes diseases of roots of seedlings of cabbage, and tobacco.
The thallus is unicellular either naked or walled, may be rounded, elongated or of various other shape. It is either holocarpic or eucarpic being differentiated into a vegetative and fertile portion. In some chytrids the thallus may develop into a rhizomycelium.
Family Synchytriaceae:
In the family Synchytriaceae the thallus is posteriorly uniflagellate and holocarpic. The thallus is at first naked, in course of growth develops a thin membrane and ultimately forms a thick-walled structure whose protoplast divides by cleavage to form a sorus of either zoosporangia or gametangia. Zoospores on being released from the zoosporangium repeat the cycle.
Whereas, the gametes by planogametic isogamy result in the development of zygote which ultimately becomes thick-walled resting sporangium. The resting sporangium on germination produces zoospores. The zoospores and the gametes are morphologically similar but their behaviour is different.
Genus Synchytrium:
Synchytrium is a very widely known chytrid. It is world-wide in distribution and several of its species are of considerable economic importance. All species are parasitic on both higher and lower plants.
They have been reported to occur in abundance on algae, mosses, ferns, and flowering plants and are rather more common on flowering plants growing in a moist environment since water is essential for their zoospores to mature, disseminate, and also to infect host cells. The intensity of infection is often correlated with environment in which the host plants are growing and usually seedlings are very susceptible.
The species which occur on flowering plants cause the development of galls on leaves, petioles, flower buds, and stems. Whereas, those which parasitize algae may cause marked local enlargement as well as elongation of the infected cell. Most terrestrial species occur on the leaves, petioles, and stems of the hosts, but some infect the roots and underground stems only.
The life cycles of the different species of Synchytrium vary considerably in length. Some are described as short-cycled and others referred to as long-cycled depending on the sequence, of development of different stages and their number. Most widely known and thoroughly investigated species is Synchytrium endobioticum which causes the destructive wart disease of potato.
The first classical study of the life history and cytology of Synchytrium endobioticum was that of Miss K. M. Curtis, published in 1921. The minute endobiotic, holocarpic thallus represented by a naked posteriorly uniflagellate zoospore with whiplash flagellum swarms in water, comes to rest on the epidermis of a young host cell at or near ground level.
It withdraws its flagellum and penetrates the host cell by a very thin naked penetration tube which pierces the cuticle and epidermal wall making a narrow channel. There is no further inward migration of the parasite in the host body. The subsequent development of the parasite is carried on in the epidermal cell originally invaded in which it remains confined.
The parasite is carried to the bottom of the epidermal cell by the streaming of protoplasm. In the meantime under the influence of the stimulus produced due to the presence of the parasite, the infected cell swells and becomes pyriform.
The neighbouring cells start dividing repeatedly (hyperplasia) followed by swelling (hypertrophy) resulting in the formation of a tumour and the surrounding epidermal cells divide similarly forming a rosette with the infected cell being placed in the middle.
The parasite along with its nucleus enlarges markedly, rounds off and forms a wall of two layers, a thick golden-brown exospore and a thin hyaline endospore, developing into a prosorus or an initial cell.
At first the prosorus is uninucleate. Soon after maturing, the prosorus germinates within the host cell. The exospore ruptures and the endospore extrudes to produce a vesicle. The cytoplasm and nucleus pass out into the vesicle consisting of extruded endospore, in this vesicle nuclear division occurs.
At this stage 5 to 7, even up to 9 multinucleate segments are delimited by the development of hyaline walls forming a sorus of segments whose identity will be determined in the later stages.
The number of nuclei in each segment increases to as many as 300 resulting from repeated nuclear division. The entire protoplast of each segment divides into as many portions as there are nuclei and each portion with its nucleus and cytoplasm develops into an uniflagellate swarmer.
Whether a segment will be a sporangium or a gametangium depends on its environmental condition. When water is in abundance the swarmer’s will behave as zoospores. Whereas, with shortage of water in the substratum the swarmer’s when released fuse with each other behaving as gametes.
The gametes originating from the same gametangium fail to fuse with each other indicating hetero- thallic condition of the gametangia. The enlarging sporangia or gametangia, as the case may be, exert pressure on all sides and are forced out of the sorus and the disorganized host cell.
The swarmer’s are set free through one or two ruptured papillae which are developed on each sporangium or gametangium. The zoospores on being liberated out from the sporangium complete the asexual cycle by inducing new infection in the host cells. The asexual cycle repeats itself several times initiating series of new infections during the same season.
In Synchytrium endobioticum with such a rapid method of reproduction taking place at a time when the potato tubers are making active growth, fresh infections are constantly taking place, and as every infection involves the formation and proliferation of rosette cells there is so much hypertrophy around infected cells that the tuber soon becomes more or less covered with the warted excrescences which are so characteristic of the wart disease of potato.
Gametic union takes place on the surface of the host or in a film of water. Karyogamy follows immediately after plasmogamy resulting in a biflagellate zygote which swarms around for a short time comes to rest on the host cell, and penetrates the host epidermal cell wall in a process similar to infection by zoospore.
The zygote sinks to the bottom of the infected cell and simultaneously with this process the surrounding cells of the host tissue divide repeatedly as a result of which a pressure is developed resulting the infected cell being buried deeply in the host tissue.
The zygote enlarges considerably, develops a three-layered thick wall and is converted into a resting sporangium. With the completion of resting period the diploid nucleus divides reductionally. Several hundred free haploid nuclei are formed by the repeated division of the haploid nuclei that are resulted from meiosis of the diploid nucleus. These free nuclei with cytoplasm subsequently form innumerable zoospores.
The thick wall of the sporangium breaks open from pressure set up by the swelling of the inner wall due to imbibition of water resulting in the bursting of the wall and the zoospores are set free. The zoospores again cause new infection on the host cells and thereby the life cycle is completed. The life cycle of the genus Synchytrium is illustrated from the species S. endobioticum (Fig. 157).
Some Indian species of Genus Synchytrium:
Synchytrium ajrekari Payak and Thirum.; S. brownii Karling; S. collapsum Syd.; S. cookii Lingappa; S. endobioticum (Schilb.) Percival.
Order Blastocladiales:
The order Blastocladiales includes both aquatic and terrestrial fungi. Some are parasitic on aquatic insects or fungi or saprophytes on dead twigs submerged in water or in soil. All are filamentous fungi with more or less well-developed mycelium. The fungi under this order exhibit fairly well-developed alternation of generations.
The vegetative body is the gametophyte which produces planogametic anisogametes. Plano gametic anisogamy leads to the development of diploid sporophytic individual which bears sessile zoosporangia. Both haploid and diploid zoospores are formed in. different zoosporangia. The haploid and diploid zoospores germinate into haploid and diploid thalli respectively.
Family Blastocladiaceae:
In the family Blastocladiaceae the thallus is attached to the substratum by rhizoids. Usually the thallus is large enough to be visible with unaided eye. The true hyphae of the vegetative body besides performing metabolic function also bear reproductive organs on their branches.
Uniflagellate gametes of two kinds, large and small, are developed in different gametangia. Gametic union takes place outside the thallus, karyogamy taking place immediately after plasmogamy resulting in the development of a diploid zygote which germinates giving rise to a diploid thallus known as sporothallus.
The diploid sporothallus bears two kinds of sporangia some of which produce haploid zoospores and others diploid zoospores. The haploid zoospores germinate to produce new vegetative body. Whereas, the development of diploid sporothallus is repeated by the germination of the diploid zoospores, thereby the life cycle is repeated.
Genus Allomyces:
It has world-wide distribution occurring in soil and on dead animal material in water. Investigations by Ralph Emerson on the isolates of Allomyces from different parts of the world have revealed valuable information on the life cycle of the different species of this fungus.
The somatic body consists of a stout trunk-like structure with the basal portion attached to the substratum by rhizoids. Numerous slender hyphal branches arise dichotomously from the distal end of the hyphal trunk.
The hyphal branches are separated into segments by pseudosepta in the form of thickened rings of cellulin at fairly regular intervals. Typical sieve-like pseudosepta resembling true cross-walls also occur in some cases.
The nature of cytoplasm is changeable, differing markedly in the same plant under altered condition of environment and at various ages- The cytoplasm is colourless except in the male gametangia being orange in colour. Allomyces is homothallic.
The gametangia appear in pairs, a male and a female, at the tips of the hyphae. The male gametangia are smaller than the female gametangia and are orange in colour due to the presence of carotene. Posteriorly uniflagellate gametes are liberated out from the gametangia by the formation of papillae on the gametangial surface.
The male gametes are smaller than the female gametes and possess conspicuous orange globule one in each. The anisogametes on being liberated out in water copulate to form a motile biflagellate zygote without having any interval between plasmogamy and karyogamy.
The motile zygote soon comes to rest and develops into a diploid thallus which is known as sporothallus. The sporothallus bears two types of sporangia; colourless thin-walled zoosporangia and reddish-brown thick-walled resistant sporangia. Both of them produce uniflagellate zoospores.
The zoospores that are set free from the thick-walled resistant sporangia develop into haploid thalli bearing paired male and female gametangia. Meiosis takes place during the development of these zoospores.
Whereas the thin-walled zoosporangia produce diploid zoospores which germinate giving rise to sporothalli bearing thin-walled zoosporangia and resistant sporangia. This process may continue indefinitely. Usually the haploid thallus occurs alternating with the diploid sporothallus exhibiting an alternation of generations.
The vegetative body of Allomyces is composed of true hyphae. It exhibits considerable differentiation of parts performing both metabolic function and bearing reproductive organs. The genus Allomyces exhibits a definite alternation of generations manifesting anisogamous sexual reproduction in its life cycle. Thus Allomyces shows advancement over the chytrids. Life cycle is presented in Figure 158.
Some Indian species of Genus Allomyces:
Allomyces anomalus Emerson; A. arbusculus Butler; A. javanicus Kniep; A. javanicus Kniep var, macrogynus Emerson.
Order Monoblepharidales:
The order Monoblepharidales includes only a few species of fungi, most of them are aquatic. This order is closely related to the Blastocladiales. The fungi belonging to this order have the outstanding characteristic of the method of sexual reproduction in which a large non-motile egg is fertilized by a small uniflagellate antherozoid. This type of sexual reproduction is not encountered in any other fungi.
Family Monoblepharidaceae:
The mycelium is saprophytic, occurring usually on decaying twigs, sometimes on animal remains lying in cool clear water. It is-fixed to the substratum by rhizoids.
The cytoplasm of the mycelium is characterized by the presence of vacuoles. These vacuoles produce foamy appearance of the mycelium. Asexual reproduction is by uniflagellate zoospores developed in an elongated zoosporangium. Sexual reproduction is oogamous. Well-organized male and female gametes are developed in antheridia and oogonia respectively.
More than one antherozoids are developed in an antheridium.
Whereas, the oogonium produces only one oosphere. The mode of fertilization is an outstanding feature of the family. A single antherozoid effects fertilization. In some cases, the fertilized oosphere pushes through the mouth of the oogonium and rounds up outside before it is transformed into an oospore.
In others, it matures within the oogonium. Germination of the oospore occurs by a germ tube during which meiosis takes place.
Genus Monoblepharis:
The genus Monoblepharis is saprophytic> primarily on submerged aquatic vegetable debris, particularly twigs, and on animal remains. The thallus consists of delicate non-septate hyphae, colourless or with a slightly brownish tinge usually protruding from the lenticels of the twigs submerged under water.
The thallus has a rhizoidal system which serves the function of anchorage to the substratum. The protoplasmic contents of the hyphae are highly vacuolated containing oil globules, which impart a characteristic foamy appearance to the hyphae.
Asexual reproduction is by posteriorly uniflagellate zoospores developed in a zoosporangium borne terminally on actively growing somatic hypha. During the development of the sporangium cytoplasm and nuclei accumulate in the end of a branch which is cut off from the rest of hyphae by a cross-wall.
The sporangial protoplast then divides into many uninucleate portions, all of which ultimately develop into posteriorly uniflagellate zoospores.
At maturity a pore is formed at the tip of the sporangium, the zoospores are released through the pore. They swim for some time, settle to a suitable substratum, become rounded, and each germinates by two germ tubes, one of which is narrowly cylindrical or somewhat irregular in shape. Many lateral sporangia may grow out behind each other giving the impression of sympodial branching.
The sporangia may also develop by the process of internal proliferation in which a new sporangium arises inside an emptied old one and grows through it by the growth of the septum through its empty membrane which is again filled with protoplast. The sporangium is cut off by a septum and the zoospores are developed from the protoplast.
The same thallus under higher temperature produces gametangia. Both antheridium and oogonium are developed in the same thallus. The antheridium is narrow-elongated structure below which is the oogonium which is larger and rounded.
The antheridium, like a zoosporangium, is cut off from the tip of a hypha by a septum. The development of antheridium and antherozoids occurs in a manner very similar to that of zoosporangium and zoospores. The antheridium produces small number of antherozoids.
The antherozoids are smaller than the zoospores, but otherwise they resemble them in appearance. The oogonium is developed from a lateral outgrowth arising from below the septum of the antheridium. A mature oogonium bears a single oosphere and has a well-defined receptive papilla on its wall. During fertilization the receptive papilla forms the point of entrance of the antherozoid.
Plasmogamy is followed immediately by karyogamy. Immediately after fertilization the fertilized oosphere may or may not be extruded from the oogonium. Whatever may be the case, after fertilization the oospore becomes thick-walled, which may be either smooth or bullate when mature.
In exogenous species of Monoblepharis where the oosphere emerges out from the oogonium, immediately after fertilization the oospore moves, with revolution and amoeboid alterations of form, to the bottom of the oogonium, and then toward the tip when it escapes.
It swims around in water by means of the flagellum. Possibly this motility is caused by the flagellum derived from the antherozoid which is thrown off rather late. It exhibits amoeboid movement, becomes spherical, and surrounds itself with a membrane which gradually thickens to form a thick wall.
In the endogenous species the oospore remains in the oogonium and development of thick wall takes place there. If the oosphere is not fertilized it is surrounded by a membrane and develops partheno- genetically to a thick-walled resting spore. During the germination of the oospore, nuclear divisions occur, in course of which meiosis takes place.
At first a hypha-like structure is produced from which ultimately a new thallus is developed. Details of the life cycle are indicated in Figure 159.
The genus Monoblepharis possesses very interesting characters for which its position in the evolutionary line of fungi is rather parallel to none.
It has resemblance with water molds for having mycelial soma, elongated hypha- like proliferating zoosporangium, and heterogametangial condition.
Again characters like, development of well-organized male and female gametes, specialized nature of fertilization, the behaviour of fertilized egg, and germination of oospore by the formation of a tube-like structure instead of producing zoospores are of special interest in this genus.
Order Saprolegniales:
The fungi included under this order are mostly inhabitants of water. Some are soil-inhabiting. They are best and widely known among the aquatic Phycomycetes and are commonly known as water molds and fish molds. They occur abundantly in all fresh-waters and usually prefer those which are clear and relatively pure.
Most of the species are saprophytes on plant and animal debris in fresh-water or in soil. A few are parasites offish and amphibians causing their serious diseases. Practically all the species may be readily grown in pure culture, either on solid media or in water cultures.
Some are parasitic on fresh-water and marine algae, especially diatoms, and on the roots of terrestrial flowering plants. All members of this order are characterized by well-developed mycelium. Biflagellate zoospores are produced in zoosporangia of various types. Sexual reproduction occurs by heterogametangial contact.
The gametangia are known as antheridia and oogonia. The oogonia containing one to several oospheres and periplasm is lacking. The entire mycelium does not take part in the development of reproductive organs. The reproductive organs are mostly terminal or subterminal.
The development of abundant mycelium and the formation of morphologically distinct oogonia and antheridia show a marked advancement of the Saprolegniales from the Chytridiales.
Again the Saprolegniales are quite distinguishable from its neighbouring order Peronosporales by the aquatic habit and persistent sporangia, whereas, the Peronosporales are characterized by a terrestrial habit and deciduous sporangia which are mostly terminal.
Some relationship can, however, be drawn between the Saprolegniales and the Peronosporales as both groups have walls containing cellulose, similar zoospore structure, and oogamous reproduction. The Leptomitales is another group of aquatic fungi showing clear similarities with the Saprolegniales.
Family Saprolegniaceae:
In members of the Saprolegniaceae the thallus is a richly branched coenocytic aggregation of eucarpic hyphae, usually with unlimited capacity for growth.
This aggregation may form a dense turf, easily visible with unaided eye on the substratum. The profusely developed mycelium consists of two portions, the rhizoidal for anchorage with the greatest diameter near the point of attachment being known as intramatrical hyphae which decrease slowly giving rise to extramatrical hyphae or the vegetative mycelium bearing reproductive organs.
The nearly cylindrical hyphae with vacuolate protoplasm lying next the wall have branching usually of the monopodial type, though rarely dichotomous. The hyphal wall unlike that of higher fungi, gives test for cellulose which turns blue with chloro-zinc-iodine. Septa are formed in the mycelium just below the reproductive organs, separating them from the somatic hyphae, which generally remain aseptate.
When the extramatrical hyphae have attained a certain age, their ends become clavate or spherical, filled with thick cytoplasm and numerous nuclei which ultimately take part in the development of biflagellate zoospores. In the meantime the clavate or spherical ends of the extramatrical hyphae are cut off by transverse septa.
The zoospores are discharged by the internal pressure and usually through the softened apex of the zoosporangium (Fig. 160B & C). Often undifferentiated masses of sport are poured out of the sporangium.
The zoosporangia are typically terminal segments of hyphae, but sometimes several are formed one behind the other. Occasionally the sporangia are formed intermingled with oogonia. Usually the zoosporangia are cylindrical, fusiform, or clavate in shape (Fig. 160A, D & E) with the exception of Pythiopsis where the sporangia are spherical to pyriform in shape.
In Aplanes sporangia are rarely produced; but when they are developed, the spores germinate in situ within the sporangium, and the germ tubes protrude through the sporangial wall. Sometimes sporangia are cut off in basipetal succession, as in Dictyuchus. Rarely intercalary sporangia are formed.
In general, the zoosporangia of the Saprolegniaceae remain permanently attached to the somatic hyphae, and even after they have discharged their zoospores. But exception is the genus Dictyuchus where the sporangia with maturity usually fall off from the somatic hyphae. Occasionally they may remain attached to the somatic hyphae. Again the sporangial wall may be persistent or evanescent.
When the sporangium has emptied its contents of spores, the basal septum grows through the empty sporangium and forms a second sporangium in the emptied space of the first sporangium. This process may be repeated several times as a result of which several sporangia may thus be formed one within the other and each maturing and shedding its spores before the next one is formed.
This sphenomenon is known as sporangial proliferation (Fig. 161 A).
The sporangial proliferation is commonly encountered in Saprolegnia and Leptolegnia. In Achlya, Aphanomyces, Pythiopsis, and Thraustotheca, the sporangia are renewed by lateral outgrowth of a portion of the hypha next to the empty sporangium and proliferation is absent.
In such cases the process may be repeated forming sympodially divided, verticillately or spirally arranged sporangia, In Isoachlya and Protoachlya cymose branching and proliferation may both occur together.
The structure and behaviour of zoospores are very variable in different genera of the Saprolegniaceae. In Saprolegnia, Isoachlya, Leptolegnia, and Leptolegniella, the zoospores are diplanetic, that is, have two motile phases (Fig. 153).
In Pythiopsis, only the primary zoospores are formed which come to rest immediately after they are liberated, shed off flagella and germinate with a hypha, the zoospores axe monoplanetic. Monoplanetism is also exhibited by Thraustotheca, but its zoospores are of secondary type.
The zoospores of Achlya and Aphanomyces are also diplanetic, but the first swarming period is very brief being confined to the sporangium and serves the purpose of carrying the spores through the terminal pore of the sporangium.
Then encyst just outside the mouth of the zoosporangium (Fig. 160C), and eventually the secondary zoospores are released from the encystments. Thus in Achlya and Aphanomyces there is a strong tendency to suppress the first swarming period of the zoospores. In Dictyuchus, no primary zoospores are liberated, instead, they encyst in the sporangium and form secondary zoospores.
The secondary zoospores escape through short exit papillae, swarm for a time and encyst (Fig. 160G & H). After a period of rest those encystments release another crop of secondary zoospores. Following the second swarming, encystment and germ tube formation occur.
Thus exhibiting a phenomenon of repeated zoospore emergence or polyplanetism (Fig. 154). Again, some zoospores may fail to escape from the sporangium, encyst within it, form germ tubes there, which pass through the openings in the sporangial wall (Fig. 160 I).
Whereas, in case of a sporangium having evanescent sporangial wall, the primary zoospores are rounded and remain aggregated together and ultimately form secondary zoospores repeatedly.
In Aplanes, the zoospore initials, while still in the sporangium, surround themselves with a wall and germinate there piercing the sporangial wall with their germ tubes. Very similar is also in Geolegnia, where the zoospore initials remain multinucleate and surround themselves with a thick wall (Fig. 160J to M) and await the decay of the sporangial wall before germinating directly to mycelium.
The sporangium is rather functionless. Both these two genera are aplanetic and they exhibit the phenomenon called aplanetism. With the suppression of the process of zoospore formation, these genera represent the culmination of an evolutionary series where degenerate asexual reproduction is possibly in favour of replacement by sexual reproduction.
It is also noteworthy that this may be the point of origin of the Subclass Zygomycetes.
At times the sporangia, after being cut off from the hyphae, may remain quiescent for a considerable period, and later discharge their spores just like normal sporangia. Such sporangia are known as resting sporangia. At the tips of certain hyphae unicellular, globoid to ovoid or pyriform, thick-walled resting spores are developed in chains which fall apart easily at the dividing septa.
After a resting period they germinate by germ tubes which either grow into hyphae or develop into short-stalked sporangia. They are termed differently by various authors as resting spores, conidia gemmae, or chlamydospores.
Sexual reproduction is oogamous. The sexual organs are developed on extramatrical hyphae, and are usually terminal in position, though occasionally intercalary. The antheridia and oogonia may be formed on the same hypha, homothallic or on different hyphae, heterothallic. But majority of the Saprolegniaceae are homothallic. The oogonia are spherical bodies containing one or more eggs inside them.
The oogonia may have smooth or pitted walls. The multinucleate antheridial branches are slender, cylindrical or clavate in form, and somewhat thicker than the hyphal branches. They are usually applied to the oogonium by their side rather than tips.
Usually one or more antheridia are applied to each oogonium. Fertilization occurs with the help of fertilization tubes, which originating from antheridia pierce through the oogonial wall and reach the oospheres.
The young oogonium is multinucleate. After nuclear division there occur degeneration of many nuclei and reorganization of the oogonial contents into a number of uninucleate eggs. In Leptolegnia, Aphanomyces, Dictyuchus, and Geolegnia, only a single egg is formed within the oogonium. In most of the remaining genera more than one eggs are produced whose number again varies from species to species.
As many as thirty eggs -may be formed in certain species.
During fertilization one nucleus of each antheridium passes through each fertilization tube into each egg and fuses with the nucleus therein. The fertilized eggs develop thick walls and are converted into oospores.
Each oospore, after a period of rest, germinates by a germ tube developed by the inner membrane of the oospore which pushes out through a crack in the outer wall. The germ tube develops directly into a new plant. It has been reported by Schrade in 1938 that meiosis occurs during oospore germination.
Genus Saprolegnia:
Saprolegnia lives in all sorts of water both saprophytically on vegetable and animal remains and parasitically on living fish, may cause epidemic by infecting fish eggs. Saprolegnia parasitica causes disease offish. Usually the infection of fish is preceded by a weakening or injury of the individuals infected.
The somatic hyphae consist of:
(i) Branched rhizoidal portion embedded in the nutritive matrix serving the purposes of anchorage and absorption of nourishment, and
(ii) Profusely branched hyphae lying outside the substratum forming cottony growth being visible even in the naked eye, some portions of which take part in the development of reproductive organs. The vegetative hyphae are coenocytic with tapering branch tips. Septa appear only at the base of the reproductive organs.
Under suitable environmental conditions the hyphal branches give rise to zoosporangia. The zoosporangia are terminal. They are elongated tapering, clavate, often slender to thicker toward the distal end. Each sporangium is separated from the rest of the hyphae by a septum laid down at the base. The sporangium is clearly distinguishable from the rest of the somatic hyphae from its dense protoplasm.
With the initiation of the sporangial development, a large number of nuclei embedded in cytoplasm stream in the hyphal branch, which is the potential sporangium, from the rest of the hyphae. This is followed by the gradual development of a septum at the base of the sporangium by which it is separated from the rest of the hyphae.
The dense mass of sporangial protoplast is then divided into as many portions as there are nuclei and each portion with cytoplasm and a nucleus develops into a spore. The whole protoplast is utilized for the development of spores. Thus, numerous primary zoospores are developed in a sporangium.
The zoospores are diplanetic. They squeeze out one by one through the terminal opening of the sporangium (Fig. 160B), swim in the surrounding water, come to rest, and encyst at a distance from the sporanginm.
After a short resting period, a thin papilla develops on the encystment, its tip dissolves, and a secondary zoospore creeps oat. The secondary zoospore after a short swarming period, encysts and the encystment germinates by a germ tube which ultimately develops into a hypha of the new individual.
Several asexual generations follow one another by sporangial proliferations (Fig. 161A). Chlamydospores or gemmae often in chains of different shapes and sizes are formed, which after a period of rest produce zoospores.
These zoospores on germination by germ tubes produce new individuals. Sexual reproduction is oogamous. When conditions are favourable, the somatic hyphae give rise to antheridia and oogonia. The oogonia are terminal on main hyphae or on lateral branches or intercalary singly or in chains.
They are spherical, oval, pyriform or sometimes fusiform bearing one to many oospheres. Each mature oosphere bears a single nucleus. The oogonial wall is smooth, papillate or often pitied and much thicker than the walls of the somatic hyphae.
The antheridia are developed on the same hypha which bears the oogonium, and arise immediately below it or on different hyphae (Fig. 161 B). They are multinucleate, slender structures borne terminally on slender branches or the somatic hyphae.
The antheridia may be short or long, simple or branched. They grow toward the oogonia and become attached to them. More than one antheridia may he attached to an oogonium. During fertilization the antheridium sends out fertilization tubes which penetrate the oogonial wall and reach the oospheres (Fig. 161C).
Upon entering the oogonium a fertilization tube often produces branches sending one branch to each oosphere.
In cases where more than one antheridia contact the oogonium, an oospher is usually approached by one fertilization tube only. The antheridium does not bear well-organized male gametes and contains a large number of nuclei embedded in a mass of cytoplasm.
The antheridial nuclei with cytoplasm are discharged from the antheridium to the oospheres through the fertilization tubes. One antheridial nucleus enters each oosphere, approaches its nucleus, and fuses with it resulting in the formation of a diploid nucleus.
Immediately after fertilization the diploid nucleus with cytoplasm is surrounded by a thick wall and an oospore is formed (Fig. 161D). The oospore is smooth-walled. After a prolonged resting period, the oospores are liberated from the oogonium by the disintegration of its wall.
The oospore germinates to produce hypha of the new individual. Meiosis of its diploid nucleus takes place during the germination of the oospore. Life cycle of the genus Saprolegnia is presented in Figure 162.
Some Indian species of Genus Saprolegnia:
Saprolegnia ferax (Gruith.) Thuret; S. monoica (Pringsh.) De Bary.
Order Peronosporales:
The order Peronosporales has reached highest point of advancement in the Oomy- cetes. The members of this order lead a terrestrial existence as parasites in the tissue of higher plants. Many of them induce destructive diseases of plants of economic importance and cause tremendous loss to crops.
Among them, mention may be made of Pythium debaryanum causes damping off of seedling disease, Phytophthora infestans causes late blight of potato disease, Albugo Candida causes white rust disease of crucifers, Peromospora pisi causes downy mildew disease of peas, Plasmopara viticola causes downy mildew disease of grape, Sclerospora graminicola causes green ear disease of Bajra, and Bremia laclucae causes downy mildew disease of lettuce.
The coenocytic profusely developed mycelium is composed of freely branched hyphae which grow intercellularly, receive nutrition from the host cells by the formation of haustoria. The hyphae may also be intracellular.
Asexual reproduction is usually accomplished by the development of biflagellate secondary zoospores borne in a sporangium. The sporangia are developed terminally or laterally on sporangiophores which may or may not be distinguishable from the somatic hyphae. The sporangiophores may again be branched or un-branched.
Depending on the environmental conditions, especially on temperature, the sporangia may also behave as conidia by directly germinating by germ tube without the development of zoospores. The production of zoospores indicates the aquatic ancestry of these fungi.
Sexual reproduction takes place by the development of oogonia and antheridia which may be borne in the same thallus or on different thalli. The oogonium contains in the centre an uninucleate oosphere and cytoplasm in the periphery which is known as periplasm. There is absence of well-organized male gametes in the antheridium.
The antheridium contains one or more antheridial nuclei embedded in cytoplasm. Fertilization is accomplished by the development of a fertilization tube through which the aniheridial nucleus or nuclei as the case may be, with cytoplasm pass into the oogonium. One antheridial nucleus fertilizes the oosphere resulting in the formation of an oospore around which smooth or variously sculptured or ornamented thick wall is developed.
The periplasm probably serves as nourishment for the developing oospore. The nature of oospore germination is either by the production of a germ tube or zoospores during which meiosis takes place.
Family Pythiaceae:
The Pythiaceae comprise fungi of wide range of habitat. The lower members are aquatic, the higher ones terrestrial, and some are amphibious intermediate species.
Many of these fungi are destructive plant pathogens. They induce serious diseases of plants of economic importance by attacking both herbaceous and woody plants.
Some of them are:
Pythium debaryanum causes damping off of seedlings, Pythium aphanidermatum causes a seedling and root-rot disease of sugar beets and radishes, also causes foot rot of papaya; Pythium ultimum causes tip blight of pea; Pythium arrhenomanes is associated with root of maize, wheat, and sugarcane; Phytophthora infestans causes late blight of potato and tomato; Phytophthora parasitica causes wilt of betel vine, Phytophthora palmivora causes bud rot of cocoanut, and fruit rot of arecanut, and Phytophthora citrophthora causes brown rot and gummosis of lemons.
Some members of the Pythiaceae attack fungi, algae and insects. There are others which form mycorrhizal component in liverworts and ferns. Many inhabit in soil. In general, members of the Pythiaceae have less parasitic tendency than their neighbouring fungi included under the family Peronosporaceae. Rather they possess saprophytic tendency than being obligate parasites.
In general, sporangia are developed at the tips of the sporangiophores represented by a sympodially branched system of hyphae which are very little differentiated from the somatic hyphae. In some species well-defined sporangiophores are developed. The sporangia may remain permanently attached to the hyphae or may fall off as soon as they are mature, deciduous.
The species with permanently attached sporangia are more primitive than those having detachable sporangia. Liberation of zoospores takes place either through a pore in the sporangial wall or by the development of a thin- walled vesicle in which imperfectly differentiated zoospores are discharged before they mature and are liberated out.
The mode of sexual reproduction in the members of the Pythiaceae follows the general pattern as indicated under the Peronosporales.
Genus Pythium:
The species of Pythium are usually encountered in soil or water, living as saprophytes on dead organic matter or as parasites upon plants causing rot of the tissues or damping off of seedlings. In Pythium debaryanum the slender mycelium composed of branching coenocytic hyphae may be both intercellular and intracellular generally does not form special haustoria penetrating the host.
Septa are formed during the development of reproductive structures.
Asexual reproduction takes place by means of biflagellate secondary zoospores produced in a sporangium which may be terminal or intercalary on the somatic hypha (Fig. 163A & B). Depending on species, the sporangium may be filamentous and scarcely distinguishable from the somatic mycelium.
It may also appear as more or less inflated mycelium with or without any definite shape, or may be distinctly spherical to oval in form (Fig. 163C & D).
Usually the tips of the somatic hyphae swell, in them protoplasm flows, and ultimately are cut off by septa from the rest of hyphae as multinucleate structures. Each sporangium then forms a beak or emission tube which is usually short but sometimes several times the diameter of the sporangium (Fig. 163 E & F).
The tip of the beak or emission tube softens and develops into a bubble-like vesicle in which passes the protoplasm of the sporangium in an undifferentiated state (Fig. 163G). The protoplasm in the vesicle becomes differentiated into biflagellate secondary zoospores (Fig. 163D & H).
The zoospores move about within the vesicle and escape singly or several in a clump by bursting of the vesicle wall (Fig. 163 I). After swimming for a while each zoospore comes to rest, encysts, and then germinates by a germ tube from which somatic hypha is developed.
In some species the sporangia germinate by the production of one to six germ tubes, e.g., Pythium ultimum, exhibiting an adaptation to a subaerial habit where there may be shortage of sufficient water for the successful distribution of zoospores.
Sporangial proliferation is absent in the genus Pythium, only exception is Pythium proliferum where sporangia are renewed by proliferation. Again sporangia are developed in basipetal chains in Pythium intermedium indicating similarity with the Albuginaceae.
Sexual reproduction is typically by the development of antheridia and oogonia which appear when the host has been killed and the fungus lives saprophytically. The oogonia and antheridia are developed on branch tips of the same hypha or of different hyphae of the same thallus (Fig. 163J & K), with some exceptions where the antheridium may be intercalary, e.g., Pythium aphanidermatum.
The hyphal tips first become inflated, multinucleate, and are set off by septa (Fig. 163L & M). As the oogonium enlarges to form a globose structure all the nuclei but one migrate into the peripheral layer where they degenerate. The centrally placed nucleus with a portion of cytoplasm forms the oosphere and the cytoplasmic layer in the periphery is the periplasm.
The number of oosphere is usually one, in some species two to six oospheres are produced, e.g., Pythium polysporum. The antheridium is also multinucleate at first but normally only one nucleus survives. The antheridium is applied at the side of the oogonium, such an antheridium is known as paragynous (Fig. 163N).
During fertilization the antheridium produces a fertilization tube which penetrates through the oogonial wall and periplasm. The male nucleus with cytoplasm passes through the fertilization tube and fertilizes the oosphere. The fertilized oosphere then forms a thick wall and becomes an oospore (Fig. 163 O). The oospore wall may be smooth or reticulate.
The oospore then goes to rest, and germinates by the production of a long germ tube during which meiosis occurs (Fig. 164). At a temperature range of 10°G.-17°G., however, the germ tube after growing up to certain length stops growing.
The protoplast of the oospore migrates through the germ tube and pushes out through the tip forming a vesicle in which secondary zoospores are developed. Life cycle of the genus Pythium is illustrated in Figure 164.
Some Indian species of Genus Pythium:
Pythium aphanidermatum (Edson) Fitzp.; P. butleri Subra- maniam; P. debaryanum Hesse.; P. graminicolum P. vexans De Bary.
Genus Phytophthora:
The genera Pythium and Phytophthora are so closely related that though the genus Phytophthora was established by De Bary (1876) later accepted by Butler (1907) and others, did not receive full support of Fitzpatrick (1923). The main differences are: the method of sporangial germination, and absence of vesicle development.
These characters do not hold good with respect to many of the species of Phytophthora indicating an unsatisfactory separation of the two genera. But it is also true that the genus Phytophthora exhibits preference for terrestrial flowering plants as hosts, and possesses looser branching of the hyphae than Pythium. The hyphae when old, become irregularly septate.
This genus is of special interest on account of its one exceedingly destructive representative, Phytophthora infestans, which occupies a historic position in Phytopathology. It is one of the earliest of parasitic fungi to receive study in any way complete or adequate, which did much to turn attention and interest toward Plant Pathology.
The genus Phytophthora contains species some of which live as saprophytes in the soil, developing as parasites in the presence of suitable host plants. Other species are usually found as ‘parasites on higher plants. Phytophthora infestans which causes the late blight of potato, tomato, and rot of potato tubers was first described in 1845 as a Botrytis.
Since then various investigators have studied different aspects of the fungus and it was fully described by De Bary in 1876.
The somatic mycelium which is composed of branched, non-septate, hyaline hyphae is both intracellular and intercellular forming rudimentary haustoria. In damp air the somatic hyphae produce abundant sporangiophores (Fig. 165A).
The sporangiophore is at first simple and bears a single apical sporangium after the production of which a lateral branch arises below the sporangium and grows on in such a way as to give the first sporangium a lateral appearance producing a sympodial type of branching with characteristic swellings at the nodes (Fig. 165B to D).
The sporangia are readily detachable from the sporangiophore and may be dispersed by rain-splash or air currents. The sporangium is thin-walled, lemon-shaped with an apical papilla (Fig. 165E). It may germinate directly by a germ tube (Fig. 165 I) to produce somatic hypha.
But most commonly the contents of the sporangium cleave to form biflagellate secondary zoospores which escape through an apical pore (Fig. 165F to H). After a few minutes the zoospores come to rest and each germinates by a germ tube producing somatic hypha.
The nature of germination of sporangia is determined by temperature and moisture.
A lower temperature is favourable for zoospore formation whereas, germination by germ tube occurs at higher temperature (Fig. 155). The genus Phytophthora differs from Pythium in the formation of zoospores in the sporangium and the discharge of zoospores through an opening at the apical papilla without the formation of a vesicle. Exception being Phytophthora palmivora, where a vesicle may at time be formed.
Phytophthora infestans when grown on culture media, the mycelium often forms chlamydospores.
Sexual reproduction takes place by the development of antheridia and oogonia.
In species of Phytophthora, e.g., P. cactorum, and P. nicotianae the nature of development and arrangement of antheridium and oogonium are essentially like Pythium debaryanum, that is, paragynous. But in others, e.g., Phytophthora infestans, P. erythroseptica, P. phaseoli, P. parasitica, P. arecae, P. himalayensis the oogonium pierces through the antheridium which takes a funnel-like appearance at the oogonial stalk.
This type of arrangement of antheridium and oogonium is known as amphigynous (Fig. 165N). In P. infestans the antheridium and oogonium always arise from the lateral branches of somatic hyphae.
They grow, and come close to each other. The young oogonium penetrates the young antheridial wall, passes into the young antheridium, grows through it, projects out, and swells to form a mature oogonium in which an oosphere is developed (Fig. 165J to M). Both antheridia and oogonia are multinucleate when young. Ultimately with maturity only one nucleus survives in each.
A mature antheridium contains only one male nucleus with cytoplasm and a mature oogonium bears an oosphere surrounded by periplasm. During fertilization due to bulging of antheridial wall, a large receptive papilla is developed.
As soon as the receptive papilla is withdrawn the antheridium produces a fertilization tube through which the antheridial nucleus with cytoplasm is brought in contact with the oosphere where karyogamy takes place resulting in the formation of an oospore (Fig. 165N).
The oospore becomes thick- walled, goes to rest, and germinates to produce a short hyphal system which terminates in a sporangium (Fig. 166). The sporangium produces biflagellate secondary zoospores which on germination produce somatic hyphae. Meiosis takes place during the germination of oospore. Life cycle of Phytophthora is presented in Figure 166.
The earlier workers were unable to find any oospore for which the existence of oospore was a much controverted point for years. Even De Bary created the genus Phytophthora on the basis of a type species in which the oospores were unknown. Clinton (1911) first obtained perfect oogonia, antheridia, and oospores of Phytophthora infestans in pure cultures.
The first report of oospore formation in nature was made by Murphy (1927) from Ireland. Barrett (1948) reported that in Phytophthora infestans oospores are developed only when two compatible strains are brought together. Oospores have recently been found in Mexico where compatible strains of the fungus co-exist.
Some Indian species:
Phytophthora arecae (Colem.) Pethybridge; P. cactorum (Leb. and Cohn) Schroet; P. colocasiae Racile; P. infestans De Bary; P. parasitica Dast var sesami Kale and Prasad.
Family Albuginaceae:
The members of this family are obligate parasites upon flowering plants. They induce white rust disease. The only genus in this family, Albugo has several species. Each species is capable of infecting a certain group of closely related host plants.
For example, Albugo Candida attacks practically all cultivated crucifers, including cabbage, radish, turnip, horse-radish, etc.; A. ipomoeae-panduranae attacks various species of the Convolvulaceae, very common on sweet potato; A. tragopogonis occurs on the Com- positae; A. portulacae on Portulaca; and A. bliti on members of the Amarantaceae.
Wilson (1907) reported that Albugo Candida infects only the crucifers, but no plants outside the family Cruciferae. In spite of the fact Thai Albugo Candida attacks so many different host plants it exhibits remarkable stability of essential characters, i.e., size of sporangia, sculpturing of oospore wall, etc.
Hence Albugo Candida is a good morphological species. But cross-inoculation from one species of host to another does not always result in infection which indicates that the species Albugo Candida is composed of a number of biological forms or strains.
These biological forms are morphologically identical, but differ in their pathological characters since their differences are detectable only in their ability to infect different cruciferous plants. The specialization of parasitism of these biological forms is known as biological specialization.
The genus Albugo is characterized by sporangia borne in chains at the tips of short, clavate and unbranched sporangiophores which are formed in clusters in a sorus.
Genus Albugo:
The genus Albugo is also commonly known as Cystopus. The name Albugo was given in 1821 and Cystopus in 1874. In view of the recommendation of the International Botanical Nomenclature the earlier name Albugo is retained and the latter name Cystopus abandoned.
Species of Albugo possess very fine coenocytic mycelium which is strictly intercellular. Small globular haustoria, developed by the hyphae pierce the host cell wall.
The hyphae ramify along the intercellular spaces and collect beneath the epidermis, branch profusely and after maturity produce numerous, short, club-shaped sporangiophores arranged in a palisade-like layer at right angles with the host epidermis (Fig. 167A). Sporangia are abstracted from the tips of the sporangiophores in basipetal succession and remain attached in rather long chains unless disturbed.
During the development of the sporangium cytoplasm accumulates in the apex of the sporangiophore, the apex becomes multinucleate and is cut off by the appearance of a septum below (Fig. 167B & G). The septum is gelatinous in nature and as the sporangium matures the gelatinous septum shrinks and forms a disjunctor whose dissolution permits the sporangium to fall off from the sporangiophore (Fig. 167D).
The entire process is repeated simultaneously with the elongation of the sporangiophore producing a chain of sporangia and disjunctors arranged alternately (Fig. 167E). Large number of sporangiophores are developed in clusters (Fig. 167A).
The sporangia are produced simultaneously in chains. Both the growth of the fungus and the production of sporangia exert a pressure from below on the host epidermis causing it to bulge producing a white blister-like appearance. The blister-like structure eventually ruptures exposing the sorus bearing sporangia and sporangiophores.
The sporangia are released from the sorus. They are disseminated by wind, water, and by other agencies as well. The sporangia are normally globose. They may appear cuboid or polyhedral by pressure during their formation. The sporangia are thin-walled, multi-nucleate and are filled with cytoplasm.
Depending on the temperature, the sporangia germinate by germ tubes or by the formation of biflagellate secondary zoospores (Fig. 167F to J), which develop into hyphae of new individuals.
Antheridia and oogonia are formed after the completion of sporangial development, when the mycelium has already invaded deep in the host tissue. They are buried in the host tissue. The details of sexual reproduction differ very little from those of Pythium debaryanum. The club-shaped multinucleate antheridium is applied to the side of the globose multinucleate oogonium (Fig. 168A).
During fertilization the oogonial wall at the point of contact of the antheridium becomes thin and a papilla develops which protrudes in the antheridium. With the disappearance of the papilla the antheridium produces a fertilization tube which passes through the thin spot of the oogonial wall and periplasm reaching the multinucleate oosphere (Fig. 168B).
A deeply granular body bearing single egg nucleus arises at the centre of the multinucleate oosphere which is known as coenocentram (Fig. 168B). The fertilization tube enters the coenocentrum, ruptures and discharges a single antheridial nucleus which fuses with the egg nucleus forming an oospore (Fig. 168C).
Immediately after fertilization the fertilization tube collapses and the coenocentrum disappears. The oospore becomes thick-walled, and variously sculptured (Fig. 168D).
The pattern of sculpturing is of taxonomic interest for the identification of species each species has a distinctive pattern of sculpturing (Fig. 168F to H). The oospore germinates by the production of biflagellate secondary zoospores and thereby behaves like a resting sporangium.
Meiosis takes place during the germination of oospore. The rough outer wall of the oospore cracks open and the inner wall grows out to form a vesicle bearing the zoospores (Fig. 168E). The zoospores disperse by the breaking of the vesicle ultimately giving rise to new individuals. Life cycle of the genus Albugo is presented in Figure 169.
Some Indian species:
Albugo bliti (Biv.) Kuntze; A. Candida (Pers.) Kuntze; A. ipomoeae-panduranae (Schw.) Swingle; A. platensis (Speg.) Swingle; A. portulacae (DC.) Kuntze.
Family Peronosporaceae:
The members of this family, commonly known as downy mildews, are obligate parasites. They induce disease of vascular plants called downy mildew.
Many of them -cause destruction of crop plants. Some of the important plant pathogens of this family are: Sclerospora graminicola on cereals, Plasmopara viticola attacks grapes, Peronospora destructor on onion, Pseudoperonospora cubensis on cucurbits, Bremia lactucae on lettuce, Peronospora tabacina on tobacco, Peronospora pisi attacks Pisum sativum, and Basidiophora entospora attacks weeds.
They possess intercellular somatic mycelium from which haustoria of various shapes are produced. The sporangiophores are distinct from the somatic hyphae and constitute the most important identifying character of the different genera. They are branched and unbranched. When branched, the sporangia are borne singly at the tips of the branchlets. The sporangia are deciduous and are disseminated by wind.
They germinate either by germ tube or by the formation of biflagellate secondary zoospores. Sexual reproduction takes place by the formation of antheridia and oogonia arising on closely adjoining hyphal tips. Mature antheridium bears a single antheridial nucleus with cytoplasm and the mature oogonium contains one uninucleate oosphere with periplasm.
Fertilization takes place by the formation of a fertilization tube resulting the formation of an oospore which becomes thick-willed and may germinate by a germ tube or by biflagellate secondary zoospores.
This family has suffered much from renaming of genera. There are five or more genera which are distinguished on the basis of their asexual characters. In Basidiophora the sporangiophores are unbranched, club-shaped, having apex covered with numerous short sterigmata each of which cuts off a single nearly spherical sporangium (Fig. 170F). In all other genera the sporangiophores are branched.
The genus Bremia -has a long sporangiophore which is dichotomously or trichotomously branched having branches branching again. The branchlets possess saucer-shaped terminal enlargements with a few sterigmata round the margin each of which bears a single sporangium (Fig. 170B).
The sporangiophores of Peronospora are also branched like Bremia but the tip of each branch tapers to a point from which the sporangium is cut off (Fig. 170C).
In Sclerospora the sporangiophore is thick, stout and sparingly branched. The branches are short and thickened producing a bushy appearance. The sporangia are borne at the tips of the branches (Fig. 170E). In Plasmopara the sporangiophores are irregularly branched.
The branches arise nearly at right angles to the main axis and the secondary branches are at right angles with the primary. The tips of the branches are truncate which bear globose to ovoid sporangia (Fig. 170A).
The genus Pseudoperonospora shows characters intermediate between Peronospora and Plasmopara and is also known as Peronoplasmopara. It possesses pseudomonopodially branched sporangiophores. The primary branch arises at acute angles with the main axis. The ultimate branchlets are acute from whose tips elliptical sporangia are developed (Fig. 170D).
Genus Peronospora:
The coenocytic branched mycelium develops along the intercellular space of the host tissue producing haustoria, which penetrate the host cells. The haustoria, in a antheridium few species, are short and knob-like, but in general they are filamentous and more or less branched. The members of this genus are aggressive parasites.
They cause serious damage to the host. The mycelium can survive from season to season in the tissues of perennating organs of the host.
Repeated dichotomously branched sporangiophores project from the host tissue usually through stomata covering the green parts of the host with a dense white growth giving a downy appearance from which the name downy mildew has been given.
The sporangia are borne singly at the acute more or less reflexed tips of the branchlets (Fig. 171 A): The sporangia are broadly elliptic to globose, blunt without any apical papilla. They are hyaline or very light- coloured. The sporangia, when mature fall and germinate directly by lateral germ tubes (Fig. 171B). Such sporangia are often termed conidia.
The antheridia and oogonia arise from closely adjoining hyphyal tips. Both of them are multinucleate when young, but with maturity become uninucleate. A mature oogonium bears an uninucleate oosphere with periplasm. So also a mature antheridium contains a single male nucleus embedded in cytoplasm (Fig. 171C).
Fertilization takes place by the development of a fertilization tube through which the antheridial nucleus comes in contact with the oosphere.
Plasmogamy and karyogamy lead to the development of an oospore which becomes thick-Walled (Fig. 171D to F), goes to rest and germinates by the formation of a germ tube. Peronospora tabacina is an exception, where oospore produces sporangial vesicle which releases biflagellate secondary zoospores. Meiosis takes place during oospore germination. The nature of life cycle of Peronospora is illustrated in Figure 172.
Some Indian Specis:
Pernospora aestivalis Syd, ex Gaum; P.brassicae Gaum; P.gaumannii Mundak; P. Pisi Syd; P.viciae (BerK) Gaum
Genus Plasmopara:
The genus Plasmopara is widely known for its species P. viticola which, besides being the causal organism of downy mildew disease of grape is associated with the discovery of the fungicide Bordeaux mixture. Bordeaux mixture was discovered by Professor Millardet, University of Bordeaux, France during 1882 and 1885 Professor Millardet got the clue of his discovery from the vineyards of Saint-Julien in Medoc, France.
While he was strolling through the vineyards he was surprised to notice that the vines growing along the path had leaves with bluish-white deposit on them and were quite healthy. Whereas, the vines growing deep in the vineyards were severely destroyed by downy mildew.
He learnt from the people of the locality that it was a custom of the vine growers of that locality to bespatter the vines beside the paths with a conspicuous poisonous-looking substance. This was a practice to discourage the passersby from pilfering the grapes. They used either verdigris—the bluestone, salts of copper or a mixture of copper sulphate and lime.
Millardet was actually looking for some substance which could be applied as a protective coating on the leaves, so that fungus spores falling on them would be destroyed before they can cause any infection to the leaves. Through experimental trials Professor Millardet discovered Bordeaux mixture, which is the most inexpensive and effective fungicide
Plasmopara produces intercellular branched coenocytic mycelium which receives nutrition from the host cells by the production of a large number of simple globose haustoria. Erect solitary or fasciculate branched sporangiophores emerge through stomata of the host (Fig. 173A). The branches arise at right angles to the main axis, so also the secondary branches.
The ultimate branches are apically obtuse which bear globose to ovoid hyaline to smoky papillate sporangia (Fig. 173A & B). The sporangia germinate either by the production of biflagellate secondary zoospores (Fig. 173C to G), or the entire protoplasmic mass escapes and then sends out a germ tube.
The oogonia and antheridia are developed rather late. Globose oogonia are formed terminally on the branches of the mycelium. A club-shaped antheridium is attached to each oogonium (Fig. 173H). During fertilization a fertilization tube is developed by the antheridium.
The details of the process of fertilization have great similarity with those of Peronospora. The oospore is thick-walled (Fig. 173 I & J). The oospore wall is composed of a smooth endospore surrounded by variously wrinkled, sometimes appearing somewhat reticulate epispore.
The oospore germinates after a period of rest by producing a hypha-like germ tube which protrudes through a small crack in the oospore wall. A sporangium is produced at the tip of the germ tube which gives rise to biflagellate secondary zoospores (Fig. 173K). Meiosis takes place during the germination of oospore. Life cycle of Plasmopara viticola is presented in Figure 174.
The Mucorales, commonly known as ‘black-molds’ or ‘pin-molds’ grow on damp organic materials, such as bread, overripe fruits, exposed foodstuffs, and animal dung.
Although most species are terrestrial saprophytes; a few species are pathogenic to man and other animals, causing diseases like bronchomycosis, and ailments of the corner, and skin; and others are of considerable importance in the decay of fruits and vegetables. Others are parasitic on other fungi, e.g., Piptocephalis and Syncephalis.
Again several species are utilized in industrial processes, such as for the manufacture of alcohols, fumaric acid, lactic acid, and certain enzymes.
The vegetative body is composed of profusely developed filamentous and richly branched mycelium. The mycelium is white in the early stage of growth and turns they or brownish with age. It is encountered lying on and in the substratum. The anycelium is differentiated into aerial hyphae and prostrate hyphae.
The prostrate hyphae serve the function of absorption (Fig. 175 A), in some species are buried in the substratum, whereas, in others remain on its surface and produce rhizoids (Fig. 175B) at certain intervals. These rhizoids perform the functions of absorption and anchorage. A connecting hypha of the two groups of rhizoids is called a stolon (Fig. 175B) which usually grows over the surface of the substratum.
Asexual Reproduction:
Reproductive bodies are usually borne on the aerial hyphae. In liquid media, particularly in presence of high concentration of sugar the hyphae may be segmented and the segments bud in yeast-like fashion. This is known as the Torula condition.
Under adverse conditions, whether drought or excess of water the hyphae become septate, and within each segment the protoplasm becomes rounded off, develops a thick wall around itself, and forms very resistant spore. Such a spore is designated as a chlamydospore (Fig. 175D).
Such a chalamydospore with the return of favourable conditions develops directly into new mycelium. The usual mode of asexual reproduction is by aplanospores which are also known as sporangiospores. These spores are produced in very large number in a sporangium (Fig. 176A). The sporangium develops at the tip of aerial hypha, the lower part of which is known as sporangiophore (Fig. 175B & G). The sporangiophore supports the sporangium.
The sporangiophores of many Mucorales are positively phototropic. Within the Mucorales there are varied forms of spores and spore-producing structures. In the Mucorales, in general, innumerable minute spores are developed in a sporangium. Whereas, in some, the spore-producing structure is much smaller in size than a typical sporangium and it produces much less number of spores.
The spores produced in it are also larger in size than those of sporangium. Such a spore-producing structure is known as a sporangiolum (pI. sporangiola) or sporangiole (p1. sporangioles). Again members of the Eniomophthorales reproduce asexually by conidium produced singly at the tip of the conidiophore arising from hyphal body produced as a result of segmentation of mycelium.
Here the conidium is structurally a one-spored sporangiolum (sporangium).
A. Evolutionary Tendencies in the Sporangial Development:
The evolutionary tendencies in the sporangial development in the Mucorales may be traced along the following lines:
(i) Series with sporangia:
The simplest and probably the most primitive type of sporangium is encountered in the genus Mortierella. Here the sporangium is the spherical apex of the sporangiophore delimited by a septum at the base (Fig. 176C).
The next step in the evolutionary line is the condition that is seen in Mucor, in whose sporangium there is a dome-shaped sterile structure in the centre known as columella (Fig. 176A), which is nothing but An arching up of the septum by further growth.
A similar type of sporangium is found in Sporodinia grandis. But here the sporangiophore is dichotomously branched (Fig. 178A). In Pilobolus there is modification of the Mucor type. In it, the columella is comparatively small and the sporangium is rather bean-shaped with its groove placed on the columella.
The sporangiophore is with an enlarged subsporangial vesicle (Fig. 176B). In Absidia, the sporangiophore widens gradually and forms a wedge-shaped base to the columella, known as apophysis.
(ii) Series with sporangiola:
Gradual development of sporangiola from sporangial condition is encountered in different members of the Mucorales. In Mucor proliferus and in some others where the sporangiophore is branched, the terminal sporangium is larger than the laterals. In Mucor proliferus the spores that are produced in the lateral sporangia are smaller in number than those produced in the terminal one.
In Thamnidium the terminal sporangium possesses a well-developed columella, whereas, the lateral sporangia are much smaller and few spored without any columella, they are best known as sporangiola (Fig. 177A).
In some species of Thamnidum, under controlled conditions the terminal does not develop at all, only the sporangiola on the lateral branches are formed.
The sporangiola that are formed by the genus Dicranophora on the lateral branches are only one-to two-spored and their spores are much larger than those of terminal sporangium which often fails to develop (Fig. 177B) whereas, the terminal sporangium is entirely lacking in Chaetocladium, suggesting a step advanced in the evolution.
A further advancement is exhibited by the development of one-spored sporangiola, which in some species are dehiscent and in others indehiscent.
These indehiscent monosporous sporangiola are considered as conidia. In the genus Blakeslea a large columella is developed in the sporangium (Fig. 177D) when it is well nourished, but the same fungus with shortage of nourishment produces smaller and few-spored sporangia without any columella—the sporangiola (Fig. 177E).
In Choanephora also both sporangia and sporangiola are formed. Again in Choanephora trispora (=Blakeslea trispora) three spores are formed in a sporangiolum (Fig. 177F, G & I). Whereas, in Choanephora cucurbitarum the sporangiola are monosporous and indehiscent behaving as conidia (Fig. 177C & H).
The genus Cunninghamella is much more advanced than the genera already mentioned. No sporangia are developed in this genus, only indehiscent sporangiola are formed which function as conidia (Fig. 177J & K).
It is evident that along with the decrease in the number of spores there is increase in the size of spores in a spore-producing structure leading to the development of one-spored indehiscent condition resembling conidial development of the Ascomycetes.
(iii) Series with merosporangia:
Another line of evolution very much parallel to the above, exhibited by the genera Syncephalis and Syncephalastrum is in the formation of numerous cylindrical sporangia—merosporangia, which are again borne either on swollen sporangiophore or terminally on branchlets of the sporangiophore (Fig. 178B to D). The spores are uniseriate in the sporangium with varying number (Fig. 178E).
The sporangial wall may be constricted showing chain of spores. Eventually, the meros- porangial peridium degenerates and the aplanospores then look like a chain of exogenous spores, or conidia. The number of aplanospores may, in some instances, be reduced to only one. A monosporous merosporangium is, in effect, a conidium.
Monos- porous sporangial condition is encountered in the genus Conidiobolus (member of the Entomophthorales). Where hyphal bodies produce conidiophores at the apex of each of which a conidium is produced. That the conidium represents monosporous sporangial condition can be shown by soaking conidium in water, when an outer peridial wall and a single ‘spore’ within it will be visible.
These instances have some resemblance with conidial development of the Ascomycetes.
B. Development of Spores:
In the Mucorales, there are three types of development of spores. In one type, the sporangial content is usually cleaved into uninucleate portions along the lines of the vacuoles using up all the protoplasm. These portions round up and after several nuclear divisions become invested with spore membranes. This condition is illustrated by species of Pilobolus.
A second type is found in Rhizopus nigricans, Phycomyces nitens, Sporodinia grandis, and others, where cleavage directly results in the formation of multinucleate masses which without undergoing nuclear division, round up, form a membrane, and spores are formed. The third type is exhibited by species of Cunning- hamella.
Here the tip of the conidiophore enlarges into a capitate structure on the surface of which short protuberances — sterigmata appear. Small globular enlargements develop at the tips of the sterigmata in which protoplasm enters and ultimately these enlargements develop into indehiscent monosporous sporangiola (conidia).
In Syncephalis and Syncephalastrum the tips of the sterigmata instead of being globular form long tube-like structures in which spores are arranged in a linear series. The sporangia, whether globular or elongated may bear one or more spores. In Blakeslea trispora the sporangia contain three spores.
Whereas, in Chaetocladium jonsii and C. brefeldii the sporangia are single-spored. Again in Cunninghamella echinulata sterigmate conidia are formed which is considered to be the highest in the line of evolution from sporangium to conidium through sporangiolum.
Sexual Reproduction:
Sexual reproduction in the Mucorales is effected by the copulation of two multinucleate gametangia which, in general, are morphologically similar—isogametangia, with a few exceptional cases in which the copulating gametangia are dissimilar-hetero- gametangia. The copulating gametangiauteay be produced by the same thallus or by different thalli.
Members of the Mucorals in which the copulating gametangia are developed on the same thallus are known as homothallic and when produced by the interaction of two different thalli they are called heterothallic.
Homothallic member is Sporodinia grandis and heterothallic members are Mucor mucedo, Rhizopus nigricans, Mucor hiemalis, Absidia spinosa, Phycomyces nitens. During the process of sexual reproduction the tips of hyphae or hyphal branches of the same thallus or different but complementary thalli on coming in contact with each other swell, and constitute progametangia (Fig. 179A).
A transverse septum develops in each progametangium to delimit it into two portions, the terminal cell thus delimited in each functions as a gametangium and the basal portion of the progametangium is known as suspensor (Fig. 179B).
The walls between the two conjugating gametangia dissolve and the contents mix resulting in the formation of a fusion cell termed coenozygote which develops to form a thick-walled structure with warty surface called zygospore (Fig. 179C & D).
When both the conjugating gametangia are morphologically similar and their entities lost as a result of conjugation the entire process is known as isogametangial copulation. But in some members, a distinct tendency of heterogametangial condition is exhibited by the unequal size of the copulating gametangia and supporting suspensors.
The product of their copulation is, however, known as zygospore since their mode of origin is very much similar to what is encountered in isogametangial condition. Heterogametangial condition is found in Zygorhynchus heterogamus (Fig. 180D & E), species of Dicranophora (Fig. 181B & C), Absidia orchidis and others. In general, the zygospore remains naked.
But exceptions arc Phycomyces nitens (Fig. 179F) and Absidia glauca (Fig. 181 A), where long outgrowths grow out from the suspensors forming a protective covering around the zygospore. Much more advanced condition is seen in Mortierella. Here a large number of short hyphae form a solid felt having its outer surface cutinized turning brown in colour, within which lies the zygospore (Fig. 180A & B).
This, Brefeld considered a true fructification. Very similar condition is found in Endogone, Where the zygospore is surrounded by a tight weft of hyphae indicating a tendency to develop a fructification (Fig. 180C).
The zygospore during its development remains along the substratum. Exceptional condition is in Phycomyces nitens, where the suspensors resemble clippers or tong-like in appearance (Fig. 179F) holding a zygospore between their tips pushing it out to a height of even 6 to 12 inches or more above the substratum. A similar condition is also exhibited by Pilobolus (Fig. 179E).
Again in Sporodinia grandis special hyphal branches arise from ordinary hyphae on which conjugating gametangia are borne. These special hyphal branches are known as zygophores (Fig. 179G). When a gametangium fails to find its copulation mate, and in some cases is surrounded by a many layered wall, it is called azygospore.
The zygospore wall usually consists of five layers. The innermost is thin granular. The next one is elastic known as cartilaginous layer which is covered by a thin sheath.
The fourth one is fragile, brown or black and is the carbonaceous layer surrounding which is the outermost layer which is either pale and elastic, or dark and fragile. The three inner layers are grouped as endospore and the two outer as exospore (Fig. 182A).
The zygospore germinates only after a long resting period. With the rupturing of the exospore, the endospore grows out to form a germ tube which develops to a mycelium-like structure known as sporangiophore, whose tip swells to form a sporangium, zygosporangium or germ sporangium (Fig. 182B). But with the shortage of nourishment a sporangium is developed directly by the endospore.
Heterothallism:
In some members of the Mucorales zygospores occur commonly in nature. Whereas, in others the formation of zygospore is rather infrequent, if not rare. The formation of zygospores in the Mucorales was for the first time observed by Ehrenberg (1820) and De Bary (1864) in Sporodinia grandis. It was assumed by earlier investigators that the zygospores are formed only under special condition of environment.
But they were unable to offer any clear explanation for the process, as such the problem remained unsolved for long time.
The mystery behind the process of zygospore formation was revealed by A. F. Blakeslee (1904) through his classical studies. He was successful in producing zygospores of Sporodinia grandis in pure culture from a single spore. But under similar conditions he failed to obtain zygospores in Rhizopus nigricans.
That really was the starting point of Blakeslee’s studies. He carried on his experiments with different members of the Mucorales like Mucor mucedo, Absidia spinosa, Phycomyces blakesleeanus, and many others.
Blakeslee, through his intensive studies showed that the production of zygospores is possible in the laboratory under cultural condition. According to him, the formation of zygospores is primarily dependent on the inherent nature of the individual and secondarily on external factors.
He found out that in some special hyphal branches of the mycelium originating from a single sporangiospore interact to form zygospores. Blakeslee called them homothallic species and the phenomenon homothallism. But the majority of species tested by him did not produce zygospores unless hyphae arising from sporangiospores of different potentialities are interacted.
To him, they are heterothallic species and the phenomenon is heterothallism.
On the basis of the mode of zygospore formation, Blakeslee subdivided the members of the Mucorales into two groups:
those which produce zygospores from the same thallus or mycelium obtained from a single sporangiospore, homothallic; and others where the zygospore formation is dependent on thalli or mycelia of two different potentialities, and zygospores can never be obtained from the mycelium arising from a single sporangiospore, heterothallic.
To the homothallic group belong: Sporodinia grandis, Dicranophora fulva, Rhizopus sexualis, Mucor genevensis, Zygorhynchus dangerdi. Whereas, Absidia caerulea, Blakeslea trispora, Choanephora cucurbitarum, Mucor mucedo, Phycomyces nitens, and Rhizopus nigricans are heterothallic.
He further explained that in heterothallic individuals production of zygospores is brought about through the interaction of mycelia of two distinct individuals which are morphologically indistinguishable, though a physiological difference evidently exists. Blakeslee labelled them (+) strain and (—) strain.
In very few exceptional cases, however, visible differences exist between the two strains, one being rather more luxuriant than the other. To the relatively well-developed strain sometimes the term (+) is applied, and the term (—) to the strain with which zygospores are produced.
According to Blakeslee, heterothallism is a condition when zygospore formation takes place by the interaction of two thalli or mycelia of different potentialities. Whereas, under homothallic condition zygospore development is completed by the same thallus and without the union of two different thalli.
He considered the terms homothallism and heterothallism corresponding to monoecism and dioecism used in higher plants. He stated that in heterothallic species, mycelium of one sex comes in contact with that of the opposite sex resulting in the development of zygospores, segregation of sex having been taken place in two different thalli.
Since there is no morphological difference between the fusing gametangia in the members of the Mucorales in which Blakeslee carried on his investigations, the interpretation of the process of zygospore development on the basis of sex as is being used in higher plants where the sexual organs are clearly distinguishable, was questioned by Blackman (1906).
According to Blackman, the application of the term sex in cases where the fusing cells are not morphologically distinguishable is not appropriate. In such cases he prefers to use the term syngamy (syngametic).
Blakleslee (1906) further emphasized that the morphological similarity of the gametangia does not rule out the possibility of differentiating (+) and (—) strains on the basis of sex.
He pointed out that the mutual indifference of two mycelia of the same sex and the active sexual reaction between mycelia of opposite sex which leads to the formation of zygospores, indicate that the strains are fundamentally different though morphologically identical.
The progametangia arise only from the stimulus of actual contact between hyphae which touch each other, and are from the very beginning adherent.
To establish the sexual identity of the (+) and the (—) strains, Blakeslee performed experiments with species having heterogametangia (Zygo- rhynchus heterogamus, Z- macrosfiorus, and Dicranophora fulva) whose larger gametangium may be considered female and the smaller as male having some analogy with the Oomycetes.
The strain reacting with the larger gametangium is to be considered male and the one reacting with the smaller gametangium female. The results were up to his expectation only with Absidia spinosa and Mucor hiemalis, both of which are hetero- thallic. But his findings could not be repeated in any other heterothallic species for which the explanation offered by him did not receive wide support.
That the difference in size of the gametangia is not constant and is sometimes controlled by the conditions of the substratum has been indicated in the heterothallic species Rhizopus nigricans, where the gametangia often differ in size indiscriminately in response to nutritional changes in the medium in which the fungus is growing.
As such the heterogametangial conditions of the Mucorales and the Oomycetes are not analogous.
Sometime later, Satina and Blakeslee (1926, 1927) attempted to indicate the difference between the two strains (+) and (—) by biochemical studies. The results they obtained were, however, not of very encouraging nature.
Their observations were based on the response of (+) and (—) strains separately to what they named Gosio reaction, which involves the capacity of the living cell to absorb and reduce salts of tellurium and selenium.
The (+) strains of Absidia blakesleana and Circinella spinosa show great capacity to reduce tellurium salts and also grow better on maltose than the (—) strains. Similarly they observed that (+) strains of Phycomyces nitens and Rhizopus migricans reduce potassium permanganate of Manolov’s reagent more vigorously than the (—) strains.
It will be more widely acceptable if the use of the terms ‘homathallic’ and ‘heterothallic’ and interptetai ion of the mechanism of zygospore formation on the basis of sex are avoided. Instead, the terms compatibility-groups or mating types, self-sterility or interfertility are used, since these terms are not ambiguous.
On the basis of compatibility, heterothallism may be explained as the phenomenon of sexual incompatibility. In a heterothallic species where the factors controlling compatibility are distributed in different thalli, no development of zygospores unless there is mating between thalli possessing factors controlling compatibility, or in other words, the thalli belonging to two compatibility groups.
The two compatible strains which are physiologically distinct are not distinguishable morphologically. Whereas, in the homothallic species zygospores are produced on a single thallus which bears the factors controlling compatibility in the same thallus.
In 1930 Ling-Young .showed that when two similar strains of Phycomyces nitens are grown in culture medium poor in nutritive substances, after a short period of growth the hyphae of the two strains fail to proceed to each other. Whereas, hyphae of two different potentialities grown under similar condition produce zygospores.
This has a resemblance with the phenomenon ‘barrage sexual’ exhibited by some Basidiomycetes between mycelia of incompatible sexual phases. This phenomenon was discovered by Vandendries and Brodie (1933).
Among the heterothallic species there are also strains in which (+) and (—) strains fail to react with each other and zygospores are not formed. These strains are called ‘neutral strains’. Similar neutrality may be induced in Mucor mucedo by prolonged cultivation of (+) and (—) strains under unfavourable conditions causing the loss of conjugation ability and developing into neutral races.
Blakeslee working with the Mucorales, reported a very interesting phenomenon of imperfect hybridization, that is, development of immature zygospores which ultimately do not mature.
Immature zygospores are formed when mating occurs (i) between unlike strains of different heterothallic species of the same or different genera or (ii) between homothallic species and both strains (+) and (—) of a heterothallic species. This special feature of the members of the Mucorales can be utilized to label the strains of the heterothallic forms in which only one strain has been obtained for use.
Sex regulation mechanism in the Mucorales:
Information obtained so far regarding the mechanism of sexual development in the Mucorales points towards a hormonal basis of sex regulation. Attempts have been made by a number of researchers to describe the specific role of hormones in sexual reproduction of the members of the Mucorales but only a few species have been studied in detail.
The sexuality of Mucor mucedo, a well-known representative of the Mucorales has been studied by several investigators, different views being expressed by them regarding the formation and nature of the sexual factor involved in the interaction between the mating systems. The sexual mechanism of M. mucedo is almost similar to that of some other members of the Mucorales.
Working on M. mucedo, M. hiemalis, and Phycomyces blakesleeanus Burgeff in 1924 reported for the first time that each strain (+) or (—) produces a diffusible or volatile substance which induces and directs the gametangial development.
The most extensive and successful studies of the regulation of the reaction have been made with M. mucedo by Plempel and his associated (1957 to 1961). Plempel postulated the existence of the two progametangia-inducing substances (termed ‘gamones’ by Plempel) produced by the respective plus and minus strains of the fungus.
Each of the two substances were found to be active towards the opposite strain. But he failed to separate them, despite repeated attempts.
These sexual factors were characterized after isolation from the mycelium of the mating strains of M. mucedo and Blakeslea trispora by Austin and others (1969). The compounds have been found to be identical with trisporic, acid B and trisporic acid C characterized earlier by Caglioti and others (1967); and Cainelli, Grasselli and Selva (1967) who isolated them from culture liquids of B. trispora.
Gooday (1968) and van den Ende (1968), however, found no indication for the existence of two mating types of progametangia-inducing substances (one active on the plus strain and one active on the minus strain) as pointed out by Plempel. Both trisporic acid B and trisporic acid C and their methyl esters were equally effective in inducing progametangia in the plus and minus mating types.
Initiation of trisporic acid production, however, occurs only when mycelia of either mating types are grown together in a culture medium. Evidently, some initiator substance(s) are believed to be responsible for primary interaction between the mating types resulting in trisporic acid formation.
These initiator substance(s) may be very labile or are rapidly metabolised. However, nothing is known definitely uptil now regarding the nature of these initiator substance(s). But it has been speculated earlier that the substance(s) concerned may be proteinaceous in nature.
It is also known that the plus strain is the major producer of trisporic acid in M. mucedo and the same strain might be the controlling partner in respect of trisporic acid production.
The above findings have been verified by Marsman using radioactive mycelia for the detection of trisporic acid. Recently Sutter reported that a compound extracted from a plus culture of B. trispora is converted into trisporic acid C when applied to the minus culture of the same fungus.
The source of the trisporic acids and their role in sexuality of some members of the Mucorales are now known. But factors affecting the biosynthesis of these trisporic acids and the individual role of each on the development of the mating systems need further investigation.
Germination of Zygospore:
The zygospore germinates only after a long resting period. The germination of a mucoraceous zygospore usually culminates in the formation of a terminal sporangium. Such a sporangium is known as a germ sporangium, also as a zygosporangium and is produced at the tip of a germ tube which develops to a mycelium-like structure also designated as germ sporangiophore.
In the germ sporangium, sporangiospores also designated as germ sporangiospores, are produced which on germination give rise to new mycelia. The germ tube which ultimately produces germ sporangiophore and germ sporangium is developed by the growth of the endospore being exposed by the rupturing of the expospore of the zygospore during germination.
But with the shortage of nourishment, a germ sporangium may be developed directly by the endospore.
The contents of the germ sporangium and the germ sporangiophore presumably represent the surviving meiotic products of zygospore germination. In majority of cases a single unbranched germ sporangiophore is produced. In Mucor hiemalis occasionally the germ sporangiophore may be branched and two germ sporangia are formed. Rarely more than one germ sporangiophore arises from a single zygospore. Zygospore may also germinate to produce vegetative mycelia.
The results obtained so far in the study of the germination of mucoraceous zygospores may be summarized as follows:
Blakeslee (1906) reported that a homothallic strain which he studied and which he designated Mucor I, gives rise to germ sporangiospores which, when isolated singly, gives rise to zygospore-producing mycelium. He designated this type of zygospore germination as pure homothallic.
Kohler (1935) in his studies on Mucor mucedo found that all of the sporangiospores obtained from a single germ sporangium give rise to mycelia which are of the same mating type. A zygospore germination of this type is known as pure heterothallic.
The germination of zygospore in Mucor hiemalis has been studied by Cutter (1942), Sjowall (1945), and Gauger (1965). They reported that the zygospores of Mucor hiemalis germinate as pure heterothallic with deviation from what is encountered in Mucor mucedo. Here the zygospores on germination produce vegetative mycelia before any .sporangiophores are produced.
Such a type of germination is referred to as a mycelial germination.
Blakeslee working on Sporodinia grandis, Mucor mucedo, Phycomyces nitens, and Rhizopus nigricans classified the possible sexual reactions exhibited by sporangiospores obtained from such germinations as being of four types.
With reference to plus (+) and minus (—) characters of the spores the four types of germination may be indicated as follows:
(i) In Sporodinia grandis which is homothallic, all the spores of the germ sporangium on germination give rise to homothallic mycelia, that is, all are (±).
(ii) In the heterothallic species Mucor mucedo, all the spores that are developed in a germ sporangium are of one strain, either all (+) or all (—). In this case the segregation of sex is completed before the germination of zygospore.
(iii) In another heterothallic species Phycomyces nitens, the germ sporangium contains three kinds of spores. They are (+), (—) and (±). This is a case of partial segregation of sex which results, besides the spores of (+) and (—) strains, in the development of some of the spares (±) which on germination give rise to homothallic mycelia.
It is most likely that some of the diploid nuclei take part in the formation of spores without undergoing meiotic division.
(iv) Blakeslee was unsuccessful in classifying the organism he called Rhizopus nigricans because of the difficulty he encountered in germinating the zygospores. But he hypothesized that a mixed type having (+) and (—) sporangiospores will be found in the same germ sporangium. This was designated by Blakeslee as the fourth type.
But Gauger (1961) was able to germinate zygospores of Rhizopus stolonifer (R. nigricans). He found that a single zygospore produces sporangiospores which, when cultured react as either all (+), all (—) or as mixtures of the two (+) and (—).
Blakeslee’s findings indicate that in the members of the Mucorales segregation of sex may take place at any stage immediately after the zygospore formation to the development of spores which varies from species to species.
Nuclear Behaviour during the Formation and Germination of Zygospore:
Cytological features associated with the formation and maturation of zygospores are not yet clearly understood due to hardness of zygospore wall, density of zygospore protoplast, presence of large amount of oil as well as granular structures in the protoplast and the nuclei being very small in size.
However, the nuclei in course of zygospore development can possibly behave in any of the following ways:
(i) Survival of only one pair of compatible nuclei which pair and ultimately fuse producing a diploid nucleus.
(ii) Pairing of all the compatible nuclei, survival of only one pair which ultimately fuse forming a diploid nucleus.
(iii) Pairing of all the compatible nuclei, conjugate division of paired nuclei, and survival of only one pair of nuclei which ultimately fuse resulting a diploid nucleus.
(iv) Pairing and fusion of all compatible nuclei producing many diploid nuclei. In this case either all the diploid nuclei survive or just one diploid nucleus survives and others degenerate.
Dangeard (1906), Moreau (1911, 1912, 1913) and Keene (1914, 1919) reported that the nuclei in the zygospore remain in pairs and ultimately fusion between the paired nuclei takes place. Lendner (1908) in Sporodinia grandis and McCormick (1912) indicated that only one pair of nuclei survives which fuse together and the rest of nuclei degenerate. Cutter (1942) made extensive studies on the Mucorales and encountered widely different conditions.
In Mucor hiemalis, Blakeslea trispora, and Absidia spivosa, within a few days of conjugation there is pairing of nuclei. Later on fusion of nuclei and meiosis of diploid nuclei take place as a result of which the dormant zygote contains haploid nuclei. In Rhizopus nigricans, Absidia glauca and Absidia coerulea some nuclei degenerate, others fuse in twos, and the dormant zygote having nuclei whose meiosis takes place immediately before the germination of the zygospore.
Family mucoraceae:
Mycelial threads are all alike or of two kinds being differentiated into aerial and some buried in the substralum. Sporangiospores are borne in a globose to pyriform sporangium. The sporangium is typically with a columella bearing many spores. In some genera the sporangia are few-spored and without columella. The sporangial wall is thin and not cutilized. Zygospores are variable, smooth or spiny.
Genera included under the family Mucoraceae are:
Pirella, Circinella, Absidia, Mycocladus, Rhizopus, Spinellus, Sporodinia, Phycomyces, Zygorhynchus and Mucor.
Genus Mucor:
This is the largest genus of the order Mucorales under the family Mucoraceae. It includes a number of species which are of widespread occurrence and considerable importance being saprophytic and parasitic on variety of substrata. It is found on almost every kind of damp material. Some species inhabit soil and hence are found on numerous soil-contaminated objects.
Others are associated with rotting fruits. A number of species are used for the manufacture of alcohol. Again the occurrence of species of Mucor on horse-dung is not uncommon.
Stout,’ well-developed, freely branched coenocytic mycelium grows loosely on substratum forming typical colonies, white in the early stages of growth and becoming coloured with the production of reproductive structures. The hyphae generally spread out evenly within and upon the substrate lacking in rhizoids and stolons.
These are nutritive hyphae. Besides these, some aerial hyphae arise from the nutritive hyphae as branches standing erect on them (Fig. 183A).
The tip of each aerial hypha swells in which cytoplasm with nuclei migrates (Fig. 183B). The tip swells considerably and the nuclei divide repeatedly. The contents of the young swollen tip divide into a central zone mainly filled with sap surrounded by a rich peripheral zone containing cytoplasm and nuclei.
The two zones (outer layer of nucleated cytoplasm, sporeplasm and inner dome-shaped area of cytoplasm, columellaplasm) are delimited by a foamy protoplasmic layer containing narrow flattened vacuoles lined one after the other (Fig. 183G & D).
These vacuoles ultimately form a continuous vacuolate layer by fusing laterally with one after the other which ultimately develops into a dome-shaped septum and the dome is known as a columella (Fig. 183E).
In the meantime cleavage of cytoplasm takes place (Fig. 183F) resulting into innumerable small uninucleate portions which round up, become invested with spore membranes, and develop into non-flagellate spores, the sporangiospores. The swollen hyphal tip ultimately develops into a sporangium in which the sporangiospores are formed (Fig. 183G).
The sporangium is large, globose and many-spored. It is supported by the rest of the aerial hypha which is called a sporangiophore (Fig. 183G).
The sporangial wall is evanescent (Fig. 183H). The sporangiospores are globose to ellipsoidal with a thin smooth wall. On liberation, they germinate into fresh mycelia. Spores may also be dispersed by bursting of the thin wall of the sporangium due to pressure that is set up in the columella exerted by the quantity of fluid that increased in the sporangiophore and columella during maturity of the sporangium.
Sexual reproduction in a heterothallic species of Mucor is effected by the copulation of two multinucleate isogametangia arising from two compatible hyphae, (+) strain and (—) strain. The gametangia may be developed either as terminal swellings of hyphae of (+) strain and (—) strain or their branches on coming in contact with each other. The process is initiated by the stimulus produced by their contact.
They swell and constitute the progametangia (Fig. 179A). Each progametangium delimits a terminal cell termed the gametangium, by a wall laid down from the edge inwards. The remainder of the progametangium is known as the suspensor (Fig. 179B).
The walls separating the two gametangia gradually dissolve from the middle toward the edge resulting a fusion cell, coenozygote, in which fusion of contents of both gametangia takes place. The entities of the two copulating gametangia are completely lost.
A thickened, several-layered wall is developed around the fusion cell which is then called a zygospore (Fig. 179C & D), in which karyogamy takes place. The zygospore germinates after remaining dormant for several months.
The thick exospore is ruptured and the endospore puts forth a germ tube, the tip of which develops into a sporangium known as a germ sporangium or zygosporangium (Fig. 182A & B). In Mucor mucedo the sporangium produces only one kind of spores (+) or (—). Occasionally the copulation between the gametangia does not take place and the zygospore fails to develop.
The gametangia are then surrounded by a many-layered wall and are known as azygospores. Life cycle of a heterothallic species of Mucor is presented in Figure 184.
Some Indian species of Genus Mucor:
Mucor hi emails. Wehmer; M. indicus Lendner; M. mucedo (L.) Fr.; M. praini Ghodat and Nechitsch; M. silvaticus Hagem.
Family Pilobolageae:
Sporangiospores are developed in a sporangium which is discoid to lenticular and many-spored. The sporangial wall is black and heavily cutinized in the upper portion. The sporangium, in most species, is provided with a subsporangial vesicle and is shot away at maturity under the influence of light. Zygospore during its growth is usually lifted up from the substratum by tong-like hyphal growth.
Genera included under the family Pilobolaceae are: Pilobolus and Pilaira.
Genus Pilobolus:
Pilobolus grows on the fresh dung of herbivorous animals, especially on horse-dung. In spite of its disagreeable habitat, Pilobolus has been studied with great interest by many investigators since it exhibits phototropic reactions. The mycelium of Pilobolus is composed of repeatedly branched coenocytic hyphae which spread in all directions through the substratum.
The part of the fungus which rises above the surface of the substratum consists of the stalk bearing the sporangium. Water drops remain adhering on the stalk glitter in the early morning sun (Fig. 185F). The stalk is about a quarter of an inch high, and swells up near the tip into a little globule, surmounted by a black cap, which is the sporangium itself.
Asexual Reproduction in Genus Pilobolus:
The fungus reproduces asexually by the development of sporangia and sporangiospores. At first the spherical sporangiophore primordium is visible to the naked eye as a minute orange bulb protruding slightly above the general level of the substratum. This bulb is cut off from the greatly enlarged end of the hypha by a transverse septum.
This is the primary bulb or the primordium from which sporangiophore and sporangium are developed (Fig. 185A). The spherical primordium is packed with minute globules of yellow oil. Then an intensely yellow branch grows up from the bulb into the air assuming upright position (Fig. 185B). This is the young sporangiophore whose tip is markedly phototropic.
The tip of growing sporangiophore then swells up into a minute spherical bulb which is destined to become the sporangium (Fig. 185C).
The swollen tip enlarges more and more to form a nearly spherical sac-like structure which is filled with protoplasm containing oil drops giving yellow colour and the sporangiophore region becomes more or less colourless.
The contents of the sac-like structure which ultimately develops into a sporangium divide to form a large number of protoplasmic fragments, each of which surrounds itself with a cell wall and becomes a spore (Fig. 185M).
At the same time the upper part of the sporangial wall begins to blacken and the subsporangial region starts to swell into a bladder-like structure which is much larger than the sporangium itself, and is known as a subsporangial vesicle (Fig. 185D to F). Often a second bladder is formed in the lower part of the sporangiophore which is anchored to the substratum by rhizoids (Fig. 185E to G).
The entire portion of the sporangiophore between the two transverse septa constitutes a water-reservoir, in which a high hydrostatic pressure is set up, so that drops of liquid often exude through the lateral wall of the sporangiophore (Fig. 185F).
Owing to this pressure, the septum lying at the base of the sporangium bulges into it forming what is known as columella (Fig. 185 I).
The sporangium is solitary, discoid to lenticular and many-spored (Fig. 185F, K & L). The wall of the sporangium itself is not uniform throughout; the upper part is thickened dark and cutinized bearing bristles with crystals of calcium oxalate, while the lower part adjoining the columella remains thin and is light-coloured (Fig. 185 I).
A mature sporangium is discharged with violence from the end of the sporangiophore. Inside the lower part of the sporangium there is a mucilaginous layer which coming in contact with water swells bursting the delicate wall so that the sporangium becomes free from the columella.
The balanced hydrostatic pressure of the columella is no longer maintained as soon as the sporangium is set free from the columella, consequently the columella bursts which results in the squirting out of a jet of fluid from the sporangiophore which has been behaving as a reservoir, and ultimately projecting the sporangium violently to a great distance (Fig. 185G, H & J) and (Fig. 308G).
Hence the fungus behaves as a thrower of missile. The sporangium so hurled sticks to any object which it happens to hit, owing to the mucilage which still clings to and also due to adhesive sporangial wall. The sporangiophores are positively phototropic.
The sporangiophore including the subsporangial vesicle contains clear watery liquid except at the base of the subsporangial vesicle where a conspicuous zone is invariably found due to accumulation of carotinoid orange pigment.
This zone appears to the naked eye as a minute orange spot at the base of the subsporangial vesicle and has been interpreted by Buller (1921) as an eye-spot, the subsporangial vesicle being re- graded as a lens focussing the light on the sensitive spot (Fig. 176B).
The subsporangial vesicle functions as a lens in much the same way as does a flask filled with water. If the rays of sun strike on one side of the vesicle they are refracted through it and converge on the opposite side forming a spot of light (Fig. 186). The protoplasm at that point thus receives a phototropic stimulus; growth and elongation occur on that side of the sporangiophore.
In consequence, the sporangium is turned toward the light until the ray strikes it head-on. A condition of physiological equilibrium is then reached and the turning movement ends. Due to phototropic movement of the sporangiophore, the sporangium tends to be directed towards open space, and in nature it is often shot forth to grass surrounding the dung.
The spores are eaten along with grass by herbivorous animals and become disseminated with the dung. Passage of the spores through the alimentary canal of the animals facilitates germination producing new individuals.
Discharge of the Sporangium:
The fact that the sporangium of Pilobolus is discharged in response to light has been thoroughly studied by many investigators like, Allen and Jolivette (1914), Parr (1918), Pringsheim and Czurda (1927), van der Wey (1929), and Buller (1934). The sporangium exhibits remarkable accuracy when light is admitted through a pin-hole.
The highest degree of accuracy is obtained with white or blue light, comparatively less with yellow light, and very inaccurate with red light.
The process was explained in greater details by Buller (1934) through his series of experiments.
According to him, the subsporangial vesicle acts as a simple eye with its lens below. When light strikes the upper surface of the sporangium the rays falling on the wall of the subsporangial vesicle bulging around the sporangium are refracted through it and converge on the opposite side below on the protoplasm and the wall at the base of the subsporangial vesicle acts as a region for light perception (Fig. 186).
At this region a photochemical change is produced including growth and elongation of the subspoiangial wall.
In response to differential light intensity, differential growth and elongation of the subsporangial wall take place which ultimately induce the movement of the subsporangial vesicle to the direction of higher light intensity resulting in the development of a curvature and a positively phototropic movement of the subsporangial vesicle.
Buller further demonstrated that the release of the sporangium from the sporangiophore takes place due to photochemical reactions on the protoplasmic contents of the sub-sporangial vesicle and result in the increase of osmotic pressure stimulating the separation of the thin sporangial wall from the columella followed by bursting of the latter with ultimate dispersal-of the sporangium from the sporangiophore.
The pressure inside the sporangiophore finally becomes so great that an explosion remits, rupture occurring along a very definite line of weakness where the columella joins the subsporangial vesicle. The elastic wall of the sporangiophore contracts almost instantaneously with a very audible click. The sporangiophore immediately after explosion shows that the line of dehiscence is a very perfect circle.
The explosion takes place so quickly that it is impossible to follow the exact details at this stage. Water ejected from the sporangiophore tears off and carries away the sporangium from the sporangiophore. The amount of water is about twice the volume of the sporangium itself. The sporangium on leaving the sporangiophore, and before it hits on object, turns upside down.
Immediately on the discharge of the sporangium the stalk region of the sporangiophore loses its rigidity and the sub-sporangial vesicle, which contracted to about half its original volume, is .brought down to the level of the substratum. The subsporangial vesicle is, however, still full of liquid, part of which is soon squeezed out. After a short time the old sporangiophore becomes completely deflated.
Buller has found using Pilobolus kleinii and P. longipes that the sporangia are shot to a maximum height of six feet and a horizontal distance of eight feet. With P. kleinii, In gold has found that sporangia may be shot to a height of 118 cm.
Sexual Reproduction in Genus Pilobolus:
Sexual reproduction takes place by the process of isogametangial copulation. The development of gametangia takes place only when there is some hindrance in the formation of sporangia. During sexual reproduction two neighbouring hyphae of the mycelium enlarge, and become club-shaped.
The swollen portions grow upright placing themselves together side by side. At the same time a large quantity of protoplasm accumulates in them.
The tips of the conjugating hyphae lie in such a position as to give the appearance of a pair of tongs (Fig. 179E). They are then cut off by transverse septa, one in each. The tips so delimited are the gametangia and the rest are the suspensors. The gametangia contain thick multinucleate protoplasm.
Their common walls are dissolved. Plasmogamy, formation of a mass of protoplasm and pairing of nuclei follow in stages. The gametangia completely fuse into a single zygospore which rests upon the two suspensors. The suspensors give the appearance of a pair of tongs (Fig. 179E).
The zygospore grows in size with a very tough and thick wall. After a long period of rest the zygospore germinates by the production of a germ tube which grows into a new mycelium. But more frequently the germ tube produces at its tip a germ sporangium, the spores of which is dispersed after maturity and germinates to produce new individuals. Meiosis is during zygospore germination.
It has been experimentally demonstrated that in P. crystallinus definite sexual strains are in existence which will alone conjugate with one another and produce zygospore. Whereas, the hyphae of the same strain will not fuse sexually among themselves and fail to form, zygospores. The results have indicated that P. crystallinus is heterothallic.
Some Indian species of Genus Pilobolus:
Pilobolus crystallinus (Wiggers) Tode; P. kleinii van Tieghem; P. longipes van Tieghem; P. minutus Speg.; P. nanus van Tieghem.
Family Choanephoraceae:
Sporangium is present in some forms, but absent in others. Sporangium when present is globose to pyriform. In some genera the sporangium is many-spored and in others it is replaced by few-spored sporangiolum or unicellular conidium covering terminal capitate enlargement of the branch of the sporangiophore or conidiophore. Zygospore is formed by gametangial copulation.
Genera included under the family Choanephoraceae are: Blakeslea, Choanephora and Cunninghamella.
Genus Cunninghamella:
The vegetative hyphae are coenocytic, branched and are differentiated into erect and prostrate portions. Sporangia are never observed in this genus. Asexual reproduction, as far as known, takes place exclusively by conidia. Conidia are borne on conidiophores which arise from vegetative hyphae. The erect vegetative hyphae are transformed into more or less branched, sometimes septate conidiophores.
Each conidiophore is terminated in a capitate vesicle covered with sterigmata bearing small, echinulate, unicellular, globose to oval or pyriform deciduous conidia (indehiscent monosporous sporangiola) (Fig. 177 J & K).
The nature of branching of the conidiophores is extremely variable—cymose to racemose. There may be a main hypha bearing a subterminal whorl of branches each of which is apically swollen. Often there is development of intercalary globose chlamydospores in the mycelium.
Almost all species are heterothallic having gametangia of approximately equal size. The formation of zygospore is very much like that in Mucor (Fig. 179A to D). The zygospore wall is rough but without appendages.
There are both parasitic and saprobic species.
Some Indian species of Genus Cunninghamella:
Cunninghamella echinulata Thaxter; C. elegans Lend.; C. intermedia Despande & Mantri; C. verticillata Pain; C. vesculosa Misra.