In this article we will discuss about the development and dehiscence of ascus and ascospores.
Development of Ascus and Ascospores:
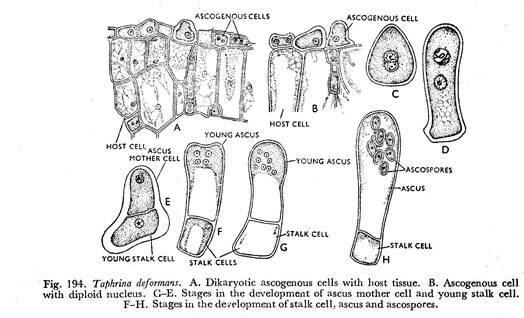
The process leading to the development of ascus and ascospores is extremely variable in the Ascomycetes. It is very simple in the genera Dipodascus, Eremascus, Schizosaccharomyces, and Saccharomyces, where the zygotic cell directly develops into an ascus. Whereas, in Taphrino deformans the dikaryotic cells of the mycelium behave as ascogenous cells (Fig. 194A).
The cells elongate breadth wise and the nuclei fuse (Fig. 194B).
The diploid nucleus divides mitotically into two daughter nuclei and the ascogenous cell also divides into two uninucleate cells. The lower one of which develops into a stalk cell and the upper one behaves as an ascus mother cell (Fig. 194C to E). The diploid nucleus of the ascus mother cell divides meiotically and mitotically to form eight daughter nuclei (Fig. 194F & G).
Soon eight ascospores are delimited centred around the nuclei in the ascus which is again developed from the ascus mother cell (Fig. 194H).
A slightly complex situation is exhibited by the genus Spermophthora where a short ascogenous hypha is formed from the top of which the ascus is developed. Again in Sphaerotheca humuli there is development of an ascogenous cell resulting from the gametangial contact.
Most complex condition is exhibited by Pyronema confluens which has attracted attention of investigators for many years. It is one of the few Ascomycetes which has been very thoroughly worked out. The study of development of ascus and ascospores in Pyronema confluens has produced many interesting points of discussion.
The study of the development of sex organs in the Ascomycetes was started by De Bary and his associates during the latter half of the nineteenth century. Of the earlier workers, mention may be made of Woronin (1866) who working or Ascophanus pulcherrimus (Ascobolus pulcherrimus Cr.) described the archicarp (ascogonium as a row of swollen cells which he termed ‘scolecite’).
It becomes enclosed in a sheath of mycelial branches, some of which he regarded as probably antheridial.
In 1871, Janezewski published a similar account of Ascobolus ferfuraceus Pers. He said that the scolecite arises as a curved multicellular branch and becomes surrounded by dense sheath of hyphae in which antheridia are present. One of the cells near the apex of the scolecite increases in size and gives rise to branches upon which asci are borne.
But they neither made critical studies of the development of asci nor paid attention to the nuclear behaviour during the process. De tails of nuclear behaviour during the development of asci and ascospores in the Ascomycetes were observed for the first time by Dangeard in 1894.
In subsequent years Harper (1895), Blackman and Fraser (1906), Welsford (1908), Brown (1909), Claussen (1912), Dodge (1912), Bagchee (1925), and Gwynne- Vaughan and Williamson (1931), and many others carried, on investigations in this line.
The sex organs in Pyronema confluens as described by De Bery consists of archicarp and antheridium produced side by side. The archicarp consists of a large globular cell known as ascogonium having a receptive neck called trichogyne at the base of which a septum is present. The antheridium is about the same length as the ascogonium but much more slender (Fig. 192A).
Both ascogonium and antheridium are multinucleate, as well as the trichogyne.
The trichogyne at first grows upright but later curves about and partially surrounds the top of the antheridium and its tip presses hard against the tip of the antheridium.
The common walls at that point and the septum at the base of the trichogyne dissolve resulting in the formation of a continuous passage through which the contents of the antheridium pass into the ascogonium and plasmogamy takes place in the ascogonium.
The ascogonium now swells up and produces innumerable outgrowths on its surface which develop into hyphal structures and are known as ascogenous hyphae (Fig. 192B).
There is some difference of opinion on the behaviour of the antheridial and ascogonial nuclei and with regard to the existence of the septum at the base of the trichogyne, the details of which will be considered in due course under cytology of ascus development. Immediately after plasmogamy the antheridial and ascogonial nuclei pair up within the ascogonium resulting in the formation of dikaryons.
The dikaryons then pass into the ascogenous hyphae (Fig. 195A).
Opinions differ as to whether they fuse in the ascogonium or merely pass in pairs in the ascogenous hyphae. The ascogenous hyphae arising from the ascogonium are aseptate when young, develop by repeated working, and subsequently become septate so that cells neighbouring the ascogonium bearing many nuclei and cells further away have only two (Figs. 192B & 195A).
The tip of each ascogenous hypha bends forming a hook and is known as crozier (Fig. 195B).
The nuclei of the crozier divide by conjugate division with spindles parallel to each other (Fig. 195G). During the division of the nuclei two septa appear between the two daughter nuclei. These two septa divide the crozier into three cells which are designated as basal or stall; cell, loop or dome cell or subterminal cell or the penultimate cell, and ultimate or tip cell (Fig. 195D).
The stalk and tip cells are uninucleate. Whereas, the loop cell is binucleate. The loop cell or the penultimate cell is the ascus mother cell. The two nuclei fuse to form a diploid nucleus in the ascus mother cell which elongates into an ascus (Fig. 195F to J). The diploid nucleus divides three times of which one is reductional to form eight haploid nuclei (Fig. 195K to N).
Each nucleus surrounds itself with a part of the cytoplasm of the ascus and develops into an ascospore which is surrounded by a rather firm wall (Fig. 195 O to Q). The development of ascospores is by free cell formation. The entire mass of cytoplasm of the ascus is not used up in the formation of ascospores.
The small portion of cytoplasm that is left in the ascus is known as the epiplasm which serves to nourish the young ascospores and also furnishes material for the sculpturing of the ascospore wall particularly in those species which have sculptured spores.
Most Ascomycetes have eight-spored asci. But asci with more than eight spores are not very rare.
In such cases more than eight Ascospores are developed in two ways:
(i) More than eight nuclei may be present as a result of successive nuclear divisions before any ascospores are delimited. In that case the final number of ascospores in each ascus is likely to approximate to some multiple of eight,
(ii) Alternatively eight ascospores are differentiated but they proceed to bud off secondary spores or conidia.
Mating Behaviour:
Ascomycetes may be homothallic or heterothallic. The basis of heterothallism is typically a single gene with two alleles, ‘A’ and ‘a’. Because of segregation during the meiotic division which precedes ascospore formation, the eight ascospores normally present in an ascus will include four of one mating type and four of the other.
Other Processes of Ascus Formation:
By the time ascus is being developed from the loop cell, the apical cell bends and touches the wall of the stalk cell. The walls at the point of contact between the tip and stalk cells may dissolve to form a dikaryotic cell which develops into an ascus (Fig. 195E to I). This is rather common in Pyronema confluens and is known as curvascous type of ascus formation.
In another case, the loop cell elongates to develop into a new crozier. The process may be continued with several times, only the last terminal loop cell develops into an ascus.
Whatever has been described above, is known as hook type or Pyronema type of ascus development. Besides this, there are different other processes of ascus formation from ascogenous hyphae or similar other structures.
They are as follows:
(i) In Geopyxis catinus, ascus development is not preceded by hook formation. Here the terminal cell of the ascogenous hypha is uninucleate, but the subterminal cell is binucleate. The binucleate subterminal cell grows out laterally to form an ascus mother cell which directly develops into an ascus, known as Geopyxis type.
(ii) In Plicaria succosa, the ascogenous hyphae are terminated by binucleate cells which directly take part in the development of asci. This is known as Plicaria type.
(iii) A synthesis between Pyronema type and Plicaria type is exhibited by Pustularia vesiculosa. In which there is development of crozier, but the loop cell of the crozier instead of developing into an ascus grows further to form a hypha whose terminal cell develops into an ascus like the Plicaria type.
(iv) Again there are Ascomycetes where asci are developed from all the binucleate cells of the ascogenous hyphae producing a chain of asci and is known as the Rectascous type.
(v) In the Laboulbeniales dikaryotic condition is achieved by the process of spermatization. The female reproductive organ itself develops into an ascogenous cell and ascogenous hyphae are absent. The ascogenous cell directly develops into an ascus. This type of ascus development is known as Laboulbenia type.
Dehiscence of Asci and Ascospore Liberation:
The mechanism of dehiscence of asci and the liberation of ascospores are extremely variable in the Ascomycetes. They are dependent upon the nature of ascocarp whether an ascocarp has hymenium wide open or the ascocarp has a pore or the ascocarp is completely closed. Again in cases where the asci are naked and not burnt in an ascocarp the mechanism of ascus dehiscence and spore liberation are also different.
In a large number of Ascomycetes the ascospores are forcibly shot out from the ascus which may rupture or open by a lid at the top to permit the ascospores to escape.
In fungi possessing ascocarp with hymenium wide open having unitunicate asci there exist the following different types of dehiscence of asci:
(i) In case of operculate ascus, the ascus opens at the top by means of a circular lid of variable size. The lid or operculum (Fig. 199B & C) remains attached at right angles or obliquely to the long axis of the ascus after the discharge of the spores. The opening formed at the top of the ascus is known as the ascostome (Fig. 199B & G).
When these asci are borne in a wide open ascocarp—apothecium, the ascospores are discharged simultaneously (Fig. 309A).
(ii) But in the inoperculate form the ascus instead of developing an operculum ruptures forming an ascostome with toothed margin produced due to discharge of spores from the ascus (Fig. 199D & E).
(iii) In case of suboperculate ascus, the ascospores are liberated through the apical pore by the displacement of pore plug.
(iv) Dehiscence of an ascus may also be by means of a slit across the top and is known as the bilabiate method (Fig. 199A).
(v) In indehiscent asci the ascospores are liberated from the asci either by deliquescing or by the brusting of the ascus wall by becoming turgid due to hydrostatic pressure from inside.
(vi) In an ascocarp with a small opening—perithecium expulsion of ascospores may be brought about by the elongation of asci so that their tips reach the opening—ostiole one by one and shoot their contents (Fig. 309B & G). Asci also break down, in some fungi, into a slimy mass in the perithecial cavity. The slimy mass containing ascospores then swells and oozes out of the ostiole and is dispersed either by insects or splashed by raindrops.
(vii) Where the asci are developed in a closed ascocarp, the ascospores are liberated by the disintegration of the ascocarp and ascus wall. Again in the hypogean Ascomycetes, the ascocarps are broken by animals and they feed upon them and the spores are dispersed through their dung.
(viii) In fungi producing naked asci, the dehiscence of asci and liberation of ascospores are, to a great extent controlled by diurnal conditions.
A bitunicate ascus normally opens by a transverse split in the cence of bituni-outer wall, a short distance below the tip, which is then pushed off cate ascus with like a thimble by the rapidly expanding elastic inner wall. The pore at the tip ascospores then escape through a pore at the tip of the inner wall of the expanded (Figs. 196F, 200, 309E—G). inner wall.