The following points highlight the three families under which ascobolus has been classified. The families are: 1. Morchellaceae 2. Pezizaceae 3. Ascobolaceae.
Family # 1. Morchellaceae:
The apothecia of this family are large and vary greatly in shape. Their receptacle may be honeycomb-like, campanulate—in both cases distinctly stipitate—or sometimes pezizoid and substipitate.
This family is characterized by its smooth elliptical ascospores, without prominent internal oil drops but bearing a number of small external guttules at their polar regions.
Genus Morchella of Morchellaceae:
The species of this genus are known as Morels or Sponge Mushrooms and are among the most delicious edible fungi known. The common edible species is Morchella esculanta having stalked apothecia about 10-15 cm high. Unfortunately, no-one has yet succeeded in cultivating any species of Morchella although cultures of mycelium are not difficult to establish on agar.
Species of Morchella usually occur under trees in open woods, orchards or in open grassland areas. They are common on moist soil rich in humus in the forests of Kashmir, Punjab, Uttar Pradesh, Nepal, and in Darjeeling in West Bengal.
Apothecia are variable in shape and size. They are scattered or rarely gregarious, erect, distinctly stipitate, morchelloid, namely with modified disc and receptacle (Fig. 205G). Disc yellowish brown to brown, covering numerous shallow depressions of sponge-like appearance on the surface of the subglobose, ovoid to narrowly conical receptacle which has either no distinct margin or with well-developed and free margin.
The depressions are separated from one another by anastomosing, sterile or rarely fertile-edged ridges, which are either honeycombed or irregular and paler than the disc, or the ridges may run more or less longitudinally in which case their edges are often darker than the disc or even almost black. The hymenium lines the shallow depressions, whilst the ridges between lack asci (Fig. 143D).
The ridge which is also known as hymenophoral trama is composed -of mostly horizontally running, septate, occasionally branched and rather large-celled hyphae. The tips of asci are curved so that the spores are projected outwards, and do not impinge on the opposite side of the depressions in which the asci are formed.
In section it can be seen that each cavity represents an apothecium and that these are compounded at the surface of the rather large, hollow pileus.
The stipe or stalk is hollow (Fig. 143D), brittle, subcylindrical, sometimes distinctly bulbous at the base, occasionally furrowed, minutely scurfy, yellowish or whitish cream.
Asci are rather subcylindrical, narrower at the rounded base, unitunicate and operculate; 8-spored, and pores are not blued in Melzer’s reagent,
Ascospores are broad elliptical to oblong elliptical, smooth-walled, hyaline to subhyaline when viewed singly but appearing cream coloured in mass, without oil globules but during their development the polar regions are crowned with numerous oil globules.
Paraphyses are stout, multiseptate near the base, branched, enlarged at the clavate apices.
Ascospores on germination produce septate hyphae which under favourable conditions give rise to ascocarp.
Some Indian species of Morchellaceae:
Morchella conica Pers.; M. esculanta (L.) Pers.; hybrida (Sow.) Pers.; M. rotunda (Pers.) Boud.
Family # 2. Pezizaceae:
The apothecia are mostly cup-, disc-, or lentil-shaped (Fig. 205G). They may be sessile or stipitate (Fig. 143A), minute to very large, bright-coloured to dark-brown. The apothecia are fleshy, waxy, leathery, cartilaginous or corneous with ectal excipulum smooth, velvety, hairy, or bristly.
 The asci are narrowly cylindrical, turn blue at least at the tip, with Melzer’s reagent; unitunicate and operculate or more rarely opening by a slit at the apex giving the open ascus a bilabiate appearance (Fig. 199A-G), occasionally marked by a thickened ring or collar near the apex. They do not protrude beyond the general level of the hymenium at maturity.
The asci are narrowly cylindrical, turn blue at least at the tip, with Melzer’s reagent; unitunicate and operculate or more rarely opening by a slit at the apex giving the open ascus a bilabiate appearance (Fig. 199A-G), occasionally marked by a thickened ring or collar near the apex. They do not protrude beyond the general level of the hymenium at maturity.
The asci bear two to many ascospores which are globose, ellipsoid or fusiform; hyaline or coloured of various shades. The ascospores are smooth or variously sculptured. The asci are mixed with filiform to clavate, hyaline or variously coloured paraphyses. The mycelium of the members of this family is septate and branched.
This family is divided into two tribes:
Pezizeae and Otideae.
Genus Peziza of Pezizaceae:
Species of Peziza grow on or beside rotting wood; on the ground, even on burnt soil; on animal dung; some partly buried in sand and others mostly on soil in woods, fields and gardens. They form more frequently saucer-shaped than cup-shaped apothecia up to 5 cm or more in diameter (Fig. 205C).
Apothecia are sessile or very short-stalked (Fig. 143A), receptacle usually thin, brittle-fleshed and often more or less scurfy externally. Some are brilliantly coloured, especially in shades of red and orange. The ascocarps are composed of tightly compacted hyphae and are typically fleshy. The hymenial layer of asci and paraphyses line the cup on the inside (Figs. 143 A and 205D).
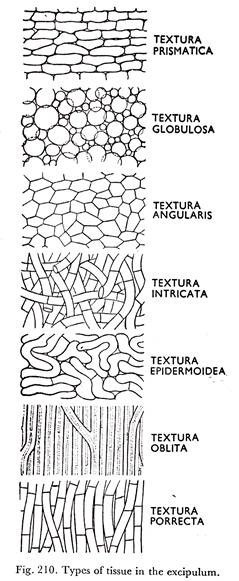
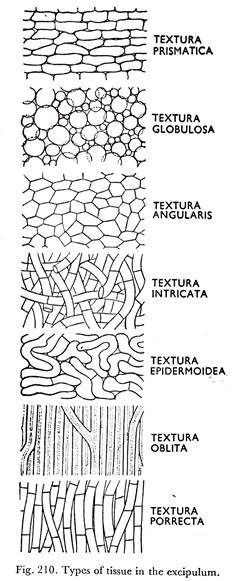
The excipular tissue is either textura globulosa or textura intricata (Figs. 205D & 210).
Asci are unitunicate and operculate; cylindric or club-shaped and nearly always contain uniseriate eight ascospores. They are blued at the tip by Melzer’s reagent. This character distinguishes Peziza from Aleuria. Aleuria aurantia is sometimes called the orange-peel Peziza. The asci do not protrude beyond the general level of the hymenium at maturity.
Ascospores are large, usually elliptical, occasionally somewhat spindle-shaped, hyaline or subhyaline to brownish with or without oil drops. The species of Peziza fall into two series, one with smooth spores without oil drops, the other with spores ornamented by warts or ridges and usually containing two large oil drops.
The second series, includes a few species whose flesh yields a coloured fluid and was formerly given generic rank under the name Galactinia. The two groups are not completely clear cut, however, and it is generally agreed that they cannot be separated genetically. The spore ornament is a valuable specific character which can be demonstrated by staining with cotton blue in lactophenol.
Paraphyses are slender, or stout, septate, unbranched, apex subclavate or clavate, often containing coloured granules.
Some Indian species of Pezizaceae:
Peziza multiguttiilata Kar & Pal; P. repanda Pers.; P. sub- cupularis Rohm; P. succosa Berk.; P. vesiculosa Bull.
Family # 3. Ascobolaceae:
The Ascobolaceae are characterized mainly by relatively broad asci which protrude above the general level of the hymenium as they mature. Ascospores commonly lie in two or three irregular rows in the ascus instead of a single vertical row. They are dark-coloured at maturity, elliptical or, if globose, there is a purplish outer layer to the spore wall soluble in alkali.
The ascospores have a double wall, a hyaline persistent inner layer and a dark brown or purple outer layer, the epispore, which commonly cracks into a characteristic pattern.
The epispore swells tip and disappears in KOH solution and dried specimens of the Ascobolaceae should never be soaked in this reagent to soften them for microscopic examination. The spores are not cohering within the ascus but are ejected singly. The apothecia are short-stalked and occur mainly on dung, a few species on burnt ground, soil or debris. They may be inconspicuously hairy to furfuraceous.
Genus Ascobolus of Ascobolaceae:
The genus Ascobolus was established by Person to include Discomycetes in which mature asci protrude from the hymenium.
Plants are generally found on dung of animals (Ascobolus scatigenus, common on dung of cow; A. minutus, on dung of cow and buffalo; A. viridis, on dung of buffalo, goat and rabbit); but often occurring on decaying plant materials (A. striisporus); on manured ground under trees (A. gollani); on burnt soil (A. carbonarius); on fabrics (A. saccoboloides) and similar other substrata.
Although saprophytic in habit, same species of Ascobolus like, Ascobolus scatigenus, A. carbonarius, A. stercorarius, and A. immetsus have been grown in pure culture to the fruiting stage.
The mycelium of the different species of Ascobolus resembles that of many other fungi. The hyphae are well-developed, profusely branched and septate. They ramify along the surface of the substratum producing a cottony appearance. When young, they are white and with age turn creamish due to accumulation of fat globules in the protoplasm.
While asexual reproduction has been reported for a few species of the genus Ascobolus, such as Ascobolus scatigenus, A stercorarius, A denudatus, and A. lignatilis; on the whole this type of reproduction is conspicuous by its absence in many of the species of the genus Ascobolus, or at least, has not been observed.
In 1905 Glaussen observed that, in Ascobolus stercorarius, great number of oidia are formed in chains from the mycelium derived from an ascospore. These oidia on germination produce mycelium which again produces oidia, but the mycelium never produces any apothecia. This has also been observed by E. S. Dowding (1931).
That the oidia are produced in several species of Ascobolus has been reported earlier by Brefeld (1891) in Ascobolus denudatus and by Falck (1902) in A. lignatilis.
J. W. Hotson (1912), B. O. Dodge (1920) and E. M. Betts (1926) have found that single-ascospore cultures of Ascobolus scatigenus (Ascobolus magnificus) never give rise to apothecia, but produce certain specialized sclerotium-like structures, the papulospores (Fig. 233).
A papulospore has one or two large central storage cell surrounded by a covering of hyphae which develop blister-like outgrowths of the storage cell. Papulospores arise in about ten days in all cultures. But they fail to produce ascocarp.
They are the spores developed by asexual method. Hotson described it as the asexual stage of Ascobolus scatigenus (Ascobolus magnificus) and named it as Papulaspora magnified Hotson. This has been confirmed by Dodge (1920), and H. G. I. Gwynne-Vaughan and H. S. Williamson (1932).
Sexual reproduction takes place by the development of antheridium and ascogonium bearing a trichogyne (Fig. 234E). The eventual product of sexual reproduction is an apothecium.
The development of an apothecium of Ascobolus scatigenus, as described by Dodge (1912, 1920) and Gwynne-Vaughan and Williamson (1932) is initiated only when two complementary mycelia are intermingled. The thallis of A. scatigenus is monoecious, that is, capable of bearing both male and female organs, but is not homothallic.
The young sexual branches are multinucleate and divide into short club-shaped multinucleate one or two celled structures, their nuclei being larger and clearer than those of vegetative hyphae.
They pass beyond rudimentary stage only when the two complementary mycelia, are intermingled. These sexual branches grow in an erect or oblique position at the surface of the medium (Fig. 234A). They may be scattered singly or associated in pairs or in groups of 3 or 4. Very frequently both structures arise at a short distance apart.
The paired branches elongate, one which can be recognised as the female branch elongates more than the other which remains erect is the male branch.
Soon both the branches become septate, the female especially, develops many septa and is differentiated into a long terminal, septate trichogyne, a large unicellular ascogonium, and a multicellular stalk. The trichogyne elongates rapidly, grows out and circles widely about the antheridium (Fig. 234B to E). The trichogyne and the antheridium fuse with each other at or near their apices.
The antheridium is plump and almost cylindrical with granular contents having large ‘lumber of nuclei. The stalk of the antheridium is made up of two to four cells. The ascogonium also contains large number of nuclei which are arranged close to the ascogonial wall, leaving a central space of reticulate or finely granular cytoplasm.
When the sex organs are fully grown, continuity is established between the contents of the antheridium and of the end cell of the trichogyne by the dissolution of walls at the point of fusion of the antheridium and the trichogyne. The nuclei begin to leave the antheridium.
They move from cell to cell through the trichogyne, lying in each cell against the proximal wall till an aperture is formed and they travel further. The opening of the wall is due to the dissolution of the central area.
After the passage of the male nuclei, the apical cell of the trichogyne becomes crushed, proximal cells retain their form, the breach in the wall is closed after the male nuclei have- passed. Later the nuclei of these cells become swollen and their contents finally disintegrate.
During the passage of the male nuclei through the trichogyne, the nuclei of the ascogonium divide. The male nuclei crowd against the final wall, it is penetrated and they pass into the ascogonium. The male nuclei reach and pair with the female nuclei which are lying in the periphery of the ascogonium.
The nuclei remaining unpaired in the ascogonium and those left behind in the antheridium and nuclei of the cells of the trichogyne, all of them disintegrate. Gywnne-Vaughan and Williamson (1932) were the first to report the passage of antheridial nuclei taking place through the-cells of the trichogyne into the ascogonium. The ascogonium increases in size and Buds out ascogenous hyphae (Fig. 234F & G).
Simultaneously with the development of the Itscogenous hyphae, sterile hyphae begin to develop from the stalk of the antheridium and ascogonial stalk to form a sheath around the antheridium, ascogonium, and the growing ascogenous hyphae (Fig. 234K).
The hyphal structures which compose the sheath may develop still below the antheridium and the ascogonium so that they insinuate themselves among the vegetative hyphae from the very beginning.
In all cases the sheath sooner or later completely encloses the ascogonium and ultimately a closed globose structure is formed. The paired nuclei pass in the ascogenous hyphae which may remain un-branched or may become dichotomously branched. The ascogenous hyphae then become septate.
The terminal cell of the ascogenous hypha forms a crozier and along with division of the paired nuclei three cells are formed, of which the binucleate penultimate cell elongates and becomes the ascus in which eight ascospores are developed, when karyogamy and meiosis take place.
Meanwhile some of the sterile hyphae grow along with the growing ascogenous hyphae giving rise to paraphyses which remain mixed with the asci in the hymenial layer of the apothecium. During the early stages of development the apothecium remains closed globose, later more or less cup-shaped and a fully mature apothecium becomes saucer-shaped (Fig. 235).
The major portion of the sterile hyphae takes part in the development of different layers of sterile tissue of the apothecium (Fig: 236).
The apothecia are sessile or substipitate, grow superficially or partially immersed in the substratum. They are soft, fleshy or waxy, usually greenish. The excipulum is of textura globulosa to angularis (Figs. 205D and 210). The ectal excipulum is smooth, furfuraceous or clothed with soft hairs.
The hypothecium is pseudoparenchymatous. The hymenium is concave, plane or convex; dotted with the ends of the asci (Fig. 236). Asci are cylindrical or clavate, unitunicate and Operculate, protruding at maturity (Fig. 237), and blued by Melzer’s reagent.
The emergent character of the asci is of very important diagnostic feature. 4-8 ascospores are borne in each ascus. The ascospores are at first hyaline becoming purple, fading to brown or blackish.
They are free in the ascus. The ascospores are ellipsoid to subglobose smooth or becoming sculptured. Spore-sculpturing is very variable, consisting of warts, ridges or crevices or longitudinally striated. Paraphyses are septate, hyaline, slender and usually adhering together, and scarcely enlarged upwards (Fig. 237). Life cycle of Ascobolus scatigenus is presented in Figure 238.
Although heterothallism has become an established fact in the Zygomycetes and higher Hymenomycetes, the acceptance of the idea of its occurrence in the Ascomycetes has not been well-established in a large number of Ascomycetes.
The hererothallic nature of Ascobolus scatigenus, A. carbonarius, A. stercorarius and A. immersus has been established by Dodge (1920), Betts (1926), Ames (1930) and Rizet (1939) respectively.
Certain principles of inheritance are much more clearly expressed by these haploid organisms than they are in diploid plants and animals. For this reason as is abundantly evident from the work of the Lindegrens, Beadle and others that such haploid organisms like the species of the genus Ascobolus specially the heterothallic ones possess immense potentialities to be used as tools for genetical studies.
Some Indian species of Genus Ascobolus of Ascobolaceae:
Ascobolus carbonarius Karst.; A. immersus Pers.; A. indicus Janwal; A. scatigenus (Berk.) Brumm.