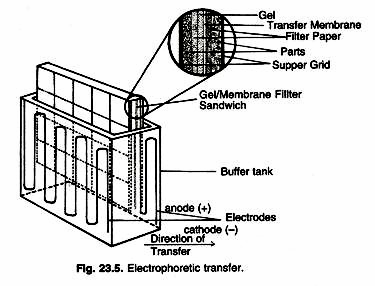
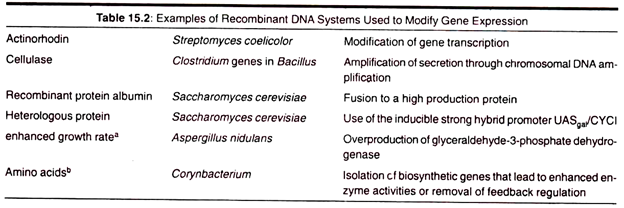
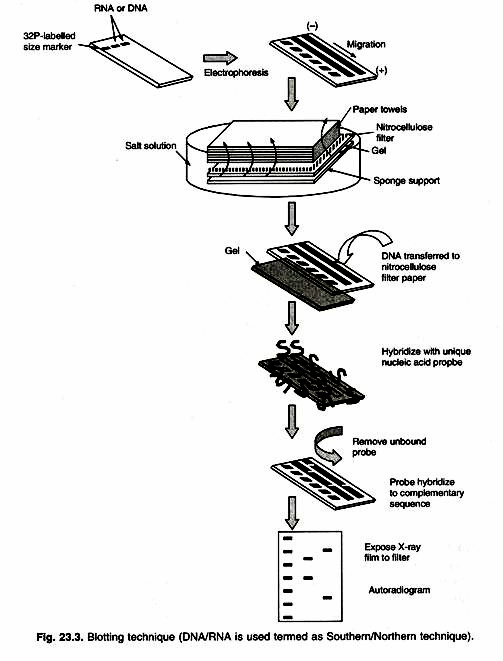
In this essay we will discuss about Industrial and Microbial Biotechnology. After reading this essay you will learn about: 1. Introduction to Industrial Biotechnology 2. Characteristics of Microbes used in Industrial Biotechnology 3. Choosing Micro-Organisms for Industrial Microbiology 4. Culture Media 5. Major Products of Industrial Biotechnology.
Contents:
- Essay on the Introduction to Industrial Biotechnology
- Essay on the Characteristics of Microbes used in Industrial Biotechnology
- Essay on Choosing Micro-Organisms for Industrial Microbiology
- Essay on Culture Media
- Essay on Major Products of Industrial Biotechnology
Contents
Essay # 1. Introduction to Industrial Biotechnology:
Industrial biotechnology, also known as white biotechnology, is the application of biotechnology for industrial purposes. It is also redefined as bioprocess technology. When our early ancestors made alcoholic beverages, they used a bioprocess.
The combination of yeast cells and nutrients (cereal grains) formed a fermentation system in which the organisms consumed the nutrients for their own growth and produced by-products (alcohol and carbon dioxide gas) that helped to make the beverage.
Bioprocess technology is an extension of ancient techniques for developing useful products by taking advantage of natural biological activities such as production of enzymes (used, for example, in food processing and waste management) and antibiotics. Use of living material offers several advantages over conventional chemical methods of production.
They usually require lower temperature, pressure, and pH. They can use renewable resources as raw materials, and greater quantities can be produced with less energy consumption. With the development of automated and computerized equipment, it is becoming much easier to accurately monitor reaction conditions and thus increases production efficiency.
As advancements in industrial technology, particularly separation and purification techniques, are made, commercial firms will be able to economically produce these substances in large amounts, and thus make them available for use in medical research, food processing, agriculture, pharmaceutical development, waste management, and numerous other fields of science and industry.
The very basis of Industrial biotechnology is microbiology. All the various production strategies bioprocess technology solely depends on various species of microbes.
Essay # 2. Characteristics of Microbes used in Industrial Biotechnology:
Microorganisms which are used for industrial production must meet certain requirements including those to be discussed below:
1. The organism must be able to grow in a simple medium and should preferably not require growth factors (i.e., pre-formed vitamins, nucleotides, and acids) outside those which may be present in the industrial medium in which it is grown.
2. The organism should be able to grow vigorously and rapidly in the medium in use. A slow growing organism no matter how efficient it is, in terms of the production of the target material, could be a liability.
3. Not only should the organism grow rapidly, but it should also produce the desired materials, whether they be cells or metabolic products, in as short a time as possible, for reasons given above.
4. Its end products should not include toxic and other undesirable materials especially if these end products are for internal consumption.
5. The organism should have a reasonable genetic, and hence physiological stability.
6. The organism should lend itself to a suitable method of product harvest at the end of the fermentation.
7. Wherever possible, organisms which have physiological requirements and protect them against competition from contaminants should be used.
8. The organism should be reasonably resistant to predators such as Bdellovibrio spp or bacteriophages.
9. Where practicable the organism should not be too highly demanding of oxygen as aeration (through greater power demand for agitation of the fermentor impellers, forced air injection, etc.) contributes about 20% of the cost of the finished product.
10. Lastly, the organism should be fairly easily amenable to genetic manipulation to enable the establishment of strains with more acceptable properties.
Essay # 3. Choosing Micro-Organisms for Industrial Microbiology:
The first step of industrial biotechnology is to find out a suitable microorganism which will be mostly fulfilling above desired characteristics.
Finding Micro-Organisms in Nature:
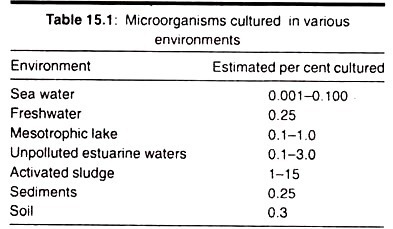
Microbes important to bioprocesses have been isolated from natural sources such as soil samples, water, and spoiled bread and fruit. Cultures from various sources were examined in order to identify strains with desirable characteristics.
Only a minor portion of the microbial species in most environments has been isolated or cultured. Due to this there is a continuing effort throughout the world to find new micro-organisms which will be useful to industrial biotechnology.
Followings are the techniques used for the development of new noble strains of microorganisms:
1. By the Process of Genetic Manipulation:
The fundamental idea behind this technique is to change the genetic makeup of the microbe in order to develop new genetic traits which will be profitable to us.
This can be achieved by following ways:
(a) Protoplast Fusion:
Protoplast fusions now widely used for the improvement of fungal organisms like yeasts and moulds. To achieve protoplast fusion protoplasts of these microbes are obtained by growing the cells in an isotonic solution followed by treating them with enzymes such as cellulose and beta-galacturonidase. This step is followed by the regeneration of protoplasts by using sucrose. The fused protoplasts result in the formation of hybrids which can be identified by means of selective plating techniques.
(b) Mutation:
Mutations, especially the one which are achieved with the help of chemical mutagens and ultraviolet light, have been very useful in developing new strains of microbes. For example, ordinary cultures of Penicilliumnotatum yields low concentrations of penicillin.
Later on a strain of Penicilliumchrysogenum (NRRL 1951) was isolated by repetitive selection procedure which was further improved through the process of mutation. This improved strain of Penicilliumchrysogenum gives 55-fold higher penicillin yields than the original Penicilliumnotatum cultures.
(c) Site directed Mutagenesis:
In the process of site directed mutagenesis short lengths of chemically synthesized DNA sequences, having the genetic information of novel traits, can be inserted into the genome of the target microorganisms. This process results in the genetic alterations of the microbe that leads to a change of one or several amino acids in the target protein.
As a consequence of this there are many changes in protein characteristics, and results in new products. These approaches are part of the field of protein engineering.
2. By the Production of Transgenic Microbes:
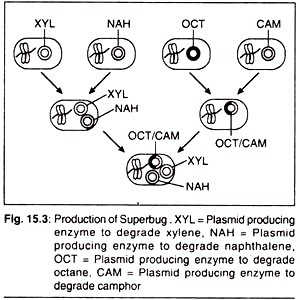
New varieties of microbes can be developed by encouraging the transfer of nucleic acids between them. This process involves the transfer of genes, having the genetic information for the synthesis of a specific product, from one organism into another. The recipient organism becomes a transgenic organism. Example of such a microbe is “Superbug” developed by A. M. Chakarabarty in 1974.
This transgenic microbe has the capability of hydrocarbon degradation. For the production of superbug he used the microbe Pseudomonas putida. It is capable of utilizing complex chemical compounds like hydrocarbons.
It is also called oil eating bug. Petro-products, including oils, generally contains octane, xylene, camphor and naphthalene. No single strain of P. putida is able to digest all these four petrochemical. Dr. Chakarabarty introduced four plasmid DNA’s from four different strains into one single cell of Pseudomonas putida and generate a superbug.
Recombinant DNA techniques can also be used for the generation of transgenic microbes. This technique has been widely exploited for the production of vaccines; for example the vaccine for the foot and mouth disease of livestock.
Genetic information for a foot-and-mouth disease virus antigen can be inserted into E. coli. This is followed by the expression of this genetic information and synthesis of the gene product for use in vaccine production.
3. By Modifying Gene Expression:
In addition to inserting new genes in organisms, it is also possible to filter the pattern of gene regulation. This is possible by means of changing gene transcription, fusing proteins, creating hybrid promoters, and by removing feedback regulation controls.
4. Preservation of Selected Strains of Micro-organisms:
Once we have selected the microorganism or virus catering to the industrial interest, then it must be preserved in its original form for any further use and research. There are different methods for microbial preservation.
Suitable methods are selected based on the:
(a) Type of micro-organism,
(b) Effect of the preservation method on the viability of micro-organism,
(c) Frequency at which the cultures are withdrawn,
(d) Size of the microbial population to be preserved,
(e) Availability of resources, and
(f) Cost of the preservation method.
Followings are some methods:
(a) Desiccation:
This involves removal of water from the culture. Desiccation is used to preserve actinomycetes (a form of fungi-like bacteria) for very long period of time. The microorganisms can be preserved by desiccating on sand, silica gel, or paper strips.
(b) Agar Slopes:
Microorganisms are grown on agar slopes in test tubes and stored at 5 to -20 °C for six months. If the surface area for growth is covered with mineral oil the micro-organisms can be stored for one year.
(c) Liquid Nitrogen:
This is the most commonly used technique to store micro-organisms for a long period. Storage takes place at temperatures of less than -196 °C and even less in vapour phase. Microorganisms are made stationary and suspended in a cryoprotective agent before storing in liquid nitrogen.
(d) Drying:
This method is especially used for sporulating microorganisms (organisms that produce spores). They Eire sterilized, inoculated, and incubated to allow microbial growth, then dried at room temperature. The resultant dry soil is stored at 4° to 5 °C.
(e) Lyophilization:
This process is also known as freeze-drying. The microbial culture is first dried under vacuum, filled in ampoules (glass vessels) then frozen. This is a most convenient technique, since it is cheap to store and easy to ship. The disadvantage is that it is difficult to open the freeze dried ampoules; also, several subcultures have to be done to restore the original characteristics of the micro-organisms.
5. Growth of Micro-organisms:
In the industrial processes the selected micro-organisms are grown using specifically designed culture media under very carefully controlled conditions such as temperature, aeration, etc.
Essay # 4. Culture Media:
Media requirements depend on the type of microorganism being used in the fermentation process, but the basic requirements remain the same, which includes:
(a) Source of energy
(b) Water, carbon source
(c) Nitrogen source
(d) Vitamins
(e) Minerals.
Designing the media for small scale laboratory purpose is relatively easy, but media for industrial purpose are difficult to prepare.
An ideal culture medium has the following features:
(a) Allow high yield of the desired product and at fast rate
(b) Allow low yield of undesired products
(c) Be sterilized easily
(d) Yield consistent products i.e., minimum batch variation
(e) Be cheap and readily available
(f) Be compatible with the fermentation process
(g) Not pose environmental problems before, during, or after the fermentation process.
The culture medium will affect the design of the fermenter. For example, hydrocarbons in the media require high oxygen content, so an air-lift fermenter should be used. Natural media ingredients are cheap but they have high batch variation. On the other hand pure ingredients (also called defined media or formulated media) have very little batch variation but are expensive.
The media should support the metabolic process of the micro-organisms and allow bio-synthesis of the desired products.
Carbon & Energy source + Nitrogen source + Nutrients → Products(s) + Carbon Dioxide + Water + Heat + Biomass
Components:
Media are designed based on the above equation using minimum components required to produce maximum product yield.
Important components of the medium are discussed as follows:
1. Carbon Sources:
Product formation is directly dependent on the rate at which the carbon source is metabolized. Also the main product of fermentation determines the type of carbon source to be used. Carbon sources include carbohydrates, oils and fats, and hydrocarbons.
(a) Carbohydrates:
These are the most commonly used carbon sources in the fermentation process. Starch is easily available carbohydrate obtained from maize, cereals, and potatoes. It is widely used in alcohol fermentation. Grains like maize are used directly in the form of ground powder as carbohydrate.
Malt and beer made from barley grains contain high concentrations of different carbohydrates like starch, sucrose, cellulose and other sugars. Sucrose is obtained from sugar cane and molasses. Molasses is one of the cheapest sources of carbohydrate.
It contains high sugar concentration and other components like nitrogenous substances and vitamins and is used in alcohol, SCP (Single-cell Protein), amino acid, and organic acid fermentations. Extraction and purification of the products is expensive.
Sulfate waste liquor is the by-product of the paper industry; it contains carbohydrates and is used in yeast cultivation. Whey is the by-product of dairy industry. It is used in alcohol, SCP, gum, vitamins, and lactic acid fermentation.
(b) Oils and Fats:
Vegetable oils are used as a carbon source. Oils provide more energy per weight compared to sugars. They also have anti-foaming properties but are generally used as additives rather than as the sole carbon source. Examples are olive oil, cotton seed oil, soya bean oil, linseed oil, and lard (animal fat).
(c) Hydrocarbons:
C12-C18 alkanes can be used as carbon sources. They are cheap, and have more carbon and energy content per weight than sugars. They can be used in organic acids, amino acids, antibiotics, enzymes, and proteins fermentation.
2. Nitrogen Sources:
Ammonia, ammonium salts, and urea are the most commonly used nitrogen sources in the fermentation process. Ammonia also serves the purpose of pH control. Other substances used as nitrogen sources are corn-steep liquor, soya meal, peanut meal, cotton seed meal, amino acids, and proteins.
3. Minerals:
Calcium, chlorine, magnesium, phosphorous, potassium and sulfur are the essential minerals for all media. Other minerals like copper, cobalt, iron, manganese, molybdenum, and zinc are needed in trace amounts and are generally present as impurities in other components. The specific concentration on these elements depends on the micro-organism being used.
4. Growth Factors:
Vitamins, amino acids, and fatty acids are used as growth factors in the fermentation process to complement the cell components of the micro-organisms.
5. Chelating Agents:
Chelating agents prevent formation of insoluble metal precipitates. They form complexes with the metal ions present in the medium and can be utilized by the micro-organisms. Chelating agents are not required in large scale fermentation processes since some of the other ingredients like yeast extract will perform the function of forming complexes with the metal ions.
One example of a chelating agent is EDTA (ethylenedi-aminetetra-acetic acid). EDTA is a versatile, being able to form six bonds with a metal ion. It is frequently used in soaps and detergents because it forms a complex with calcium and magnesium ions.
These ions are in hard water and interfere with the cleaning action of soaps and detergents. Other chelating agents are citric acid and pyrophosphates
6. Buffers:
Buffers are used to maintain the pH of the medium as microbial growth is affected by the pH changes. Optimum pH for most microorganisms is 7.0. Commonly used buffers are calcium carbonate, ammonia, and sodium hydroxide.
7. Antifoaming Agents:
Microbial process produces a large amount of foam in the fermentation vessel. This is due to microbial proteins or other components of the media. Foaming causes removal of cells from the media and their autolysis, thus, releasing more microbial foam-producing proteins, hence, aggravating the problem.
Foam will reduce the working volume in the fermentation vessel, decrease rate of heat transfer, and deposit cells on the top of the fermenter.
The air filter exits then become wet allowing growth of contaminating microorganisms. Antifoaming agents are also called surfactant, i.e., they reduce the surface tension in the foam and destabilize the foam producing proteins. Commonly used antifoaming agents are stearyl alcohol, cotton seed oil, linseed oil, olive oil, castor oil, soya bean oil, cod liver oil, silicones, and sulphonates.
8. Air:
Air is required for aeration and is supplied to the fermenter by means of pumps or compressors. It is sterilized by passing through filters before being introduced. The amount of air required and the extent of purity depends on the fermentation process being carried out.
9. Steam:
Steam is used to sterilize fermenters and other equipment and to control temperature. Continuous dry steam supply is required for the fermentation process, and care should be taken to prevent condensation.
Factors that Influence Microbial Growth:
Temperature, water, pH, and nutrients can influence microbial growth.
(a) Temperature:
Most microorganisms that are mesophiles require temperatures of 25-40°C for optimum growth. As temperatures increase or decrease the growth rate is adversely affected. Thermophiles require high temperatures over 50°C; they cannot survive at low temperatures. Psychrophiles require very low temperatures -15 to 20°C for survival and growth.
(b) Water:
Microorganisms require optimum amounts of water to maintain their metabolism and produce required products.
(c) pH:
Optimum pH for bacteria is 6.5-7.5, yeasts 4-5, moulds 4-7. The pH of the medium should be maintained at optimum level for the micro-organism being employed to ensure better product yield.
(d) Nutrients:
Microorganisms require optimum concentrations of nutrients like nitrogen, vitamins, and minerals for maximum growth rate. This will be different for each of the different types. The optimum concentration of nitrogen is 0.1-1mg/L.
Culture Procedures:
1. Sterilization:
The media and culture vessel are sterilized in order to prevent the growth of any unwanted microorganisms which contaminates our desired culture. Sterilization is carried out in many ways. The most widely method of sterilization is done by the help of an autoclave which is a type of steam sterilization.
An autoclave or pressure cooker maintains a temperature of 120°C for 15 to 20 minutes under 15 psi pressures. Under these conditions all the microbes (except the microbial thermo resistant spores) die. When microbes are cultivated in a fermentor in the industries then it is convenient to sterilize the fermentor first as a whole before letting the media to enter it.
2. Maintaining Suitable Environment for Microbial Growth:
There are various factors which influence the growth of the microorganisms. This includes the composition of the growth medium, concentration of salts, pH, temperature, level of aeration, etc. Most of the bacteria grow at neutral pH, whereas yeast and fungi prefer acidic pH.
3. Aeration and Mixing:
Mixing of the broth is essential for the uniform distribution of the nutrients in the culture medium. Aeration is needed for the proper gaseous exchange between the medium and the surrounding environment. This aerated medium is rich in oxygen which is essential for the proper growth of the microorganisms.
Aeration and mixing is achieved by shaking the medium on a shaker in the case of small scale cultures whereas in big bioreactors it is achieved by stirring the medium with the help of a mechanical stirrer with baffles attached to it.
Equipment’s Necessary for Microbial Culture at Industrial Scale:
Followings are the various equipment’s which are necessary for culturing microorganisms in the laboratory.
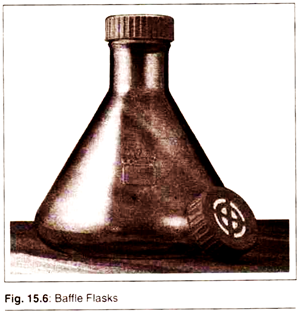
1. Baffle Flasks:
Baffle flasks are a type of modified flasks for microbial culture. In such flasks there are v-shaped notches in the sides. The presence of baffles promotes the level of oxygen transfer to the growing culture and thereby boosts the growth of microbes.
2. Stirred Tank Fermenters:
These are the most commonly used fermenters. They are cylindrical vessels with a motor driven agitator to stir the contents in the tank. The Top-entry stirrer (agitator) model is most commonly used because it has many advantages like ease of operation, reliability, and robustness. The Bottom-entry stirrer (agitator) model is rarely used.
Laboratory scale stirred tank fermenters are made of borosilicate glass with a stainless steel lid and top-entry stirrer. Typical volume of these fermenters is 1 to 100 litres. Stirrers consist of a motor attached to the shaft.
The shaft contains impellers. Stainless steel fermenters are also used in laboratories and have special requirements. They should be made of high grade stainless steel, have an internal surface that should be polished to reduce adhesion of contents to the walls of the fermenter, and have joints that should be smooth and free from pin-holes.
Fermentation Vessel Additional Equipment:
Agitator:
This consists of shaft, impellers with 4 to 6 blades and motor to drive. Shafts should have double seals to prevent leakage of the contents. The main function of the agitator is mixing of the contents, aeration, and removal of carbon dioxide produced during fermentation process by mixing action.
Different types of impellers are:
1. Rushton blade or disc turbine: This is the most commonly used impeller because of its simple design, robustness and ease of operation. It has 4 to 6 blades.
2. Open turbine impellers
3. Marine impellers.
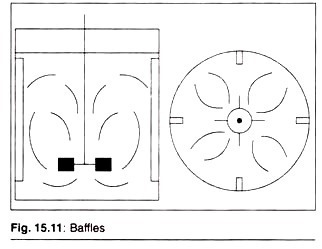
Baffles:
Four baffles are fixed on the walls of the fermenter which are used to prevent formation of a vortex. Impellers and baffles produce axial or radial flow patterns of the contents in the fermenter.
Compressor:
Compressors are used to pump air under pressure into the fermenter from the bottom, to promote aeration and mixing. They are used mainly in the large fermenters. For laboratory scale, small fermenters air pumps are used. Compressors should be oil-free in order to provide food-grade clean air to the fermenter.
Filter:
This is used to filter the air supplied by the compressor before it enters the fermenter to ensure clean air. Types of filters are:
1. Membrane Filters:
They are made of cellulose acetate and nitrate with constant pore size of 0.2 mm to ensure filtering of particulate matter. They are not cheap but easy to maintain.
2. Packed Bed Filters:
They contain glass wool or cotton wool packed in a container through which air is passed. Pore size is not uniform and these filters are susceptible to compaction and wetting thus causing channeling.
3. Cartridge Filters:
Filter element is present in a stainless steel or polycarbonate cartridge. They are more reliable and durable but are expensive.
Sparger:
Filtered air is introduced to the fermenter from the bottom through a sparger in the form of small air bubbles to ensure proper aeration and mixing.
Sensors:
These are used to monitor and control various fermentation parameters like pH, temperature and oxygen content.
Other Equipment for Fermenting:
Autoclave:
Autoclaves are used to sterilize equipment, media, and other components of fermentation. They are similar to pressure cookers. Different sizes of autoclaves are available on the market.
Ovens:
Hot air ovens are used to sterilize or dry the equipment used in the fermentation process.
The equipment to be dried or sterilized should withstand high temperatures, borosilicate or Pyrex glass equipment being good examples. The inner chamber is made of heat resistant stainless steel and has a fan to circulate the hot air evenly throughout the chamber to ensure proper heat transfer. Microwave ovens are used to perform drying and melting agar. The main advantage of microwave ovens is they take a very short time to do the job.
Incubators:
Incubators are used to cultivate micro-organisms from stock cultures to produce inoculum. Incubators provide optimum temperature for growth of micro-organisms.
Pumps:
Liquid/media is introduced to the fermenter by a pump. There are various types of pumps available, including:
1. Peristaltic:
They provide constant flow rate and mild pumping. Liquids do not back- flow, so check-valves are not required
2. Mini or Delta:
They are used for adding pH control agents like acid, alkali, and adding anti-foam agents. Mini pumps are fixed-speed pumps and can pump against the pressure.
3. Larger Pump:
They are used to add nutrients to the medium. Speed can be varied by altering the bore size of the tubes.
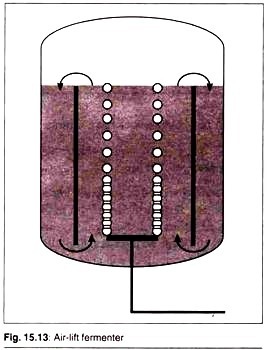
Air-Lift Fermenters:
These fermenters do not have mechanical agitation systems (motor, shaft, impeller blades) but contents are agitated by injecting air from the bottom. Sterile atmospheric air is used if microorganisms are aerobic and “inert gas” is used if micro-organisms are anaerobic.
This is a gentle method of mixing the contents and is most suitable for fermentation of animal and plant cell cultures since the mechanical agitation produces high shearing stress that may damage the cells.
Air-lift fermenters are most widely used for large-scale production of monoclonal antibodies. Draft tubes are used in some cases to provide better mixing, mass transfer, and to reduce bubble coalescence by inducing circulatory motion.
Fixed Bed Fermenters:
These are also called immobilized cell fermenters. The cells are absorbed onto or entrapped in the solid surfaces like plastic beads, glass or plastic wool and solidified gels to render them immobile. Fixed bed fermenters are most commonly used for waste water treatment and as biological filters in small aquarium water recycling systems and production of amino acids and enzymes.
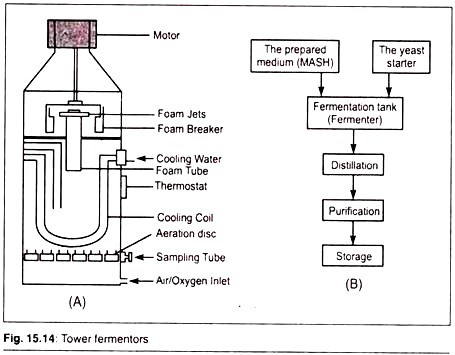
Tower Fermenters:
Tower fermenters are simple in design and easy to construct. They consist of a long cylindrical vessel with an inlet at the bottom, an exhaust at the top, and a jacket to control temperature. They do not require agitation hence there are no shafts, impellers or blades.Tower fermenters are used for continuous fermentation of beer, yeast and SCP.
Batch Culture Fermentation:
This type of fermentation is also called a closed culture system because nutrients and other components are added in specific amounts at the start of the process and are not replenished once the fermentation has started.
At the end of the process the product is recovered; then, the fermenter is cleaned, sterilized, and used for another batch process. In the initial stages microorganisms grow at a rapid rate in the presence of excess nutrients but as they multiply in large numbers they use up the nutrients. They also produce toxic metabolites which retard further growth of microorganisms during the later stages of the fermentation process.
Fed-Batch Culture:
In this process the nutrients and substrates are added at the start of the process and at regular intervals after the start. This is called controlled feeding. Inoculum is added to the fermentation vessel when micro-organisms are in exponential growth phase. Fed-batch culture is controlled by feed-back control and control without feed-back.
1. Feed-Back Control:
The fermentation process is controlled by monitoring process parameters like dissolved oxygen content, carbon dioxide to oxygen ratio, pH, concentration of substrate, and concentration of the product.
2. Control without Feed-Back:
The substrates and nutrients are added at regular intervals. Fed-batch culture requires special equipment such as a reservoir which holds the nutrients, pH modifiers so that they can be added to the fermenter at regular intervals, and pumps to deliver culture medium aseptically to the fermenter.
Continuous Culture Fermentation:
This method prolongs the exponential growth phase of microbial growth as nutrients are continually supplied and metabolites and other wastes are continually removed thus promoting continual growth of the microorganisms. Continuous culture fermentation is advantageous because of its high productivity. Two control methods are used in continuous culture fermentation, namely, chemostat and turbidostat.
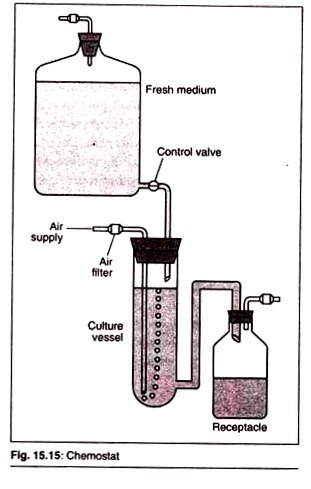
Chemo-stat:
This medium contains excess of all but one of the nutrients which determine the rate of growth of the microorganism. At steady state of the chemo-stat the rate of input of medium into the fermenter is equal to the rate of output of the fermenter.
Turbidostat:
This medium contains excess of all nutrients, so the microbial growth is at its maximum specific growth rate. The system consists of a photoelectric cell which is a turbidity sensor that detects changes in turbidity of the contents in the fermenter and then controls the amount of medium fed to the fermenter.
Measurement and Kinetics of Microbial Growth:
The growth of a microorganism may result in the production of a range of metabolites but to produce a particular metabolite the desired organism must be grown under precise cultural conditions at a particular growth rate.
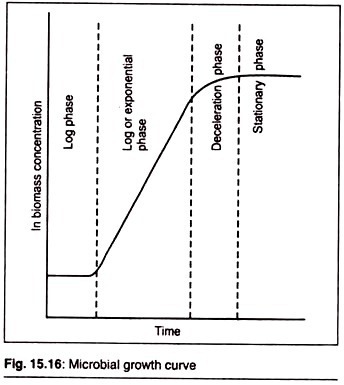
If a micro-organism is introduced into a nutrient medium that supports its growth, the inoculated culture will pass through a number of stages and the system is termed batch culture. Initially, growth does not occur and this period is referred to as the lag phase and may be considered a period of adaptation.
Following an interval during which the growth rate of the cells gradually increases, the cells grow at a constant, maximum rate and this period is referred to as the log or exponential phase, which may be described by the equation
dx/dt = nx (1)
where x is the cell concentration (mg ml-1), t is the time of incubation (h), and p the specific growth rate (h1). On integration equation (1) gives
xt = x0 eπt (2)
where x0 is the cell concentration at time zero and xt is the cell concentration after a time interval, t h.Thus, a plot of the natural logarithm of the cell concentration against time gives a straight line, the slope of which equals the specific growth rate.
The specific growth rate during the exponential phase is the maximum for the prevailing conditions and is described as the maximum specific growth rate. Equations (1) and (2) ignore the facts that growth results in the depletion of nutrients and the accumulation of toxic by-products and thus predict that growth continues indefinitely.
However, in reality, as substrate (nutrient) is exhausted and toxic products accumulate, the growth rate of the cells deviates from the maximum and eventually growth ceases and the culture enters the stationary phase. After a further period of time, the culture enters the death phase and the number of viable cells declines.
It should be remembered that this description refers to the behaviour of both unicellular and mycelial (filamentous) organisms in batch culture, the growth of the latter resulting in the exponential addition of viable biomass to the mycelial body rather than the production of separate, discrete unicells.
As already stated, the cessation of growth in a batch culture may be due to the exhaustion of a nutrient component or the accumulation of a toxic product. However, provided that the growth medium is designed such that growth is limited by the availability of a medium component, growth may be extended by addition of an aliquot of fresh medium to the vessel.
If the fresh medium is added continuously, at an appropriate rate, and the culture vessel is fitted with an overflow device, such that culture is displaced by the incoming fresh medium, a continuous culture may be established.
The growth of the cells in a continuous culture of this type is controlled by the availability of the growth limiting chemical component of the medium and, thus, the system is described as a chemostat. In this system a steady-state is eventually achieved and the loss of biomass via the overflow is replaced by cell growth.
The flow of medium through the system is described by the term dilution rate, D, which is equal to the rate of addition of medium divided by the working volume of the culture vessel.
The balance between growth of cells and their loss from the system may be described as:
dx/dt – growth – output
dx/dt = µx – Dx
Under steady-state conditions,
dx/dt = 0
and, therefore, px = Dx and p = D. Hence, the growth rate of the organisms is controlled by the dilution rate, which is an experimental variable. It will be recalled that under batch culture conditions an organism will grow at its maximum specific growth rate and, therefore, it is obvious that a continuous culture may be operated only at dilution rates below the maximum specific growth rate.
Thus, within certain limits, the dilution rate may be used to control the growth rate of a chemo-stat culture.
The mechanism underlying the controlling effect of the dilution rate is essentially the relationship between p, specific growth rate, and s, the limiting substrate concentration in the chemo-stat, demonstrated by Monod in 1942:
π = πmax s/(Ks +s) (3)
where Ks is the utilization or saturation constant, which is numerically equal to the substrate concentration when p is half pmax. At steady-state, p = D, and, therefore,
D = πmas s̅/(Ks + s̅)
where S is the steady-state concentration of substrate in the chemo-stat, and
s̅ = KsD/ ( πmas – D) (4)
Equation (4) predicts that the substrate concentration is determined by the dilution rate.
In effect, this occurs by growth of the cells depleting the substrate to a concentration that supports that growth rate equal to the dilution rate.
If substrate is depleted below the level that supports the growth rate dictated by the dilution rate the following sequence of events takes place:
(a) The growth rate of the cells will be less than the dilution rate and they will be washed out of the vessel at a rate greater than they are being produced, resulting in a decrease in biomass concentration.
(b) The substrate concentration in the vessel will rise because fewer cells are left in the vessel to consume it.
(c) The increased substrate concentration in the vessel will result in the cells growing at a rate greater than the dilution rate and biomass concentration will increase.
(d) The steady-state will be re-established.
Product Recovery:
In batch culture fermentation microorganisms grow initially and at the end of fermentation. The resultant broth contains product and dead microorganisms, thus it is essential to separate out the desired product. Microorganisms can be removed from the broth by filtration or centrifugation to separate microbial cell from the cell-free solution containing the product.
The product is isolated from cell-free solution by precipitation, adsorption, or solvent extraction techniques.
Precipitation:
This is the simplest method of isolation. Fermentation products like carbohydrates are precipitated by adding alcohol, while organic acids are salted out of the solution.
Solvent Extraction:
This technique provides isolation and purification of product in a single step. Organic solvent is added to the fermentation broth which dissolves the product to form a solvent layer which is separated from the aqueous layer and is evaporated to obtain the pure product.
Ion Exchange:
Ion exchange resins containing organic complexes exchange their ion for the product. The process involves two steps, adsorption and elution.
1. Adsorption:
The fermentation broth when passed through a column containing the resin gets absorbed to the resin.
2. Elution:
Product is separated from the resin by passing suitable reagent through the column. The advantage of this technique is reusability of the ion-exchange resin after the product is isolated.
Essay # 5. Major Products of Industrial Biotechnology:
There are many products which are produced in the industries by the help of fermentation technology.
These are discussed as follows:
Antibiotics:
Many antibiotics are produced by the help of microorganisms such as actinomycetes (e.g., Streptomyces) and filamentous fungi. Following are some antibiotics which are produced by the help of fermentation technology.
History:
While our scientific knowledge of antibiotics has only recently been developed, the practical application of antibiotics has existed for centuries. The first known, use was by the Chinese about 2,500 years ago. During this time, they discovered that applying the mouldy curd of soybeans to infections had certain therapeutic benefits.
It was so effective that it became a standard treatment. Evidence suggests that other cultures used antibiotic-type substances as therapeutic agents.
The Sudanese-Nubian civilization used a type of tetracycline antibiotic as early as 350 A.D. In Europe during the Middle Ages, crude plant extracts and cheese curds were also used to fight infection. Although these cultures used antibiotics, the general principles of antibiotic action were not understood until the twentieth century.
The development of modern antibiotics depended on a few key individuals who demonstrated to the world that materials derived from microorganisms could be used to cure infectious diseases. One of the first pioneers in this field was Louis Pasteur. In 1877, he and an associate discovered that the growth of disease-causing anthrax bacteria could be inhibited by a saprophytic bacteria.
They showed that large amounts of anthrax bacilli could be given to animals with no adverse-affects as long as the saprophytic bacilli were also given. Over the next few years, other observations supported the fact that some bacterially derived materials could prevent the growth of disease-causing bacteria.
In 1928, Alexander Fleming made one of the most important contributions to the field of antibiotics. In an experiment, he found that a strain of green Penicilliummould inhibited the growth of bacteria on an agar plate. This led to the development of the first modern era antibiotic, penicillin.
A few years later in 1932,. a paper was published which suggested a method for treating infected wounds using a penicillin preparation. Although these early samples of penicillin were functional, they were not reliable and further refinements were needed. These improvements came in the early 1940s when Howard Florey and associates discovered a new strain of Penicillium, which produced high yields of penicillin. This allowed large-scale production of penicillin, which helped launch the modern antibiotics industry.
After the discovery of penicillin, other antibiotics were sought. In 1939, work began on the isolation of potential antibiotic products from the soil bacteria streptomyces. It was around this time that the term antibiotic was introduced.
Selman Waxman and associates discovered streptomycin in 1944. Subsequent studies resulted in the discovery of a host of new, different antibiotics including actinomycin, streptothricin, and neomycin all produced by Streptomyces.
Other antibiotics that have been discovered since include bacitracin, polymyxin, viomycin, chloramphenicol and tetracyclines. Since the 1970s, most new antibiotics have been synthetic modifications of naturally occurring antibiotics.
Raw Materials:
The compounds that make the fermentation broth are the primary raw materials required for antibiotic production. This broth is an aqueous solution made up of all of the ingredients necessary for the proliferation of the microorganisms.
Typically, it contains a carbon source like molasses, or soy meal, both of which are made up of lactose and glucose sugars. These materials are needed as a food source for the organisms. Nitrogen is another necessary compound in the metabolic cycles of the organisms. For this reason, an ammonia salt is typically used.
Additionally, trace elements needed for the proper growth of the antibiotic-producing organisms are included. These are components such as phosphorus, sulfur, magnesium, zinc, iron, and copper introduced through water soluble salts. To prevent foaming during fermentation, anti-foaming agents such as lard oil, octadecanol, and silicones are used.
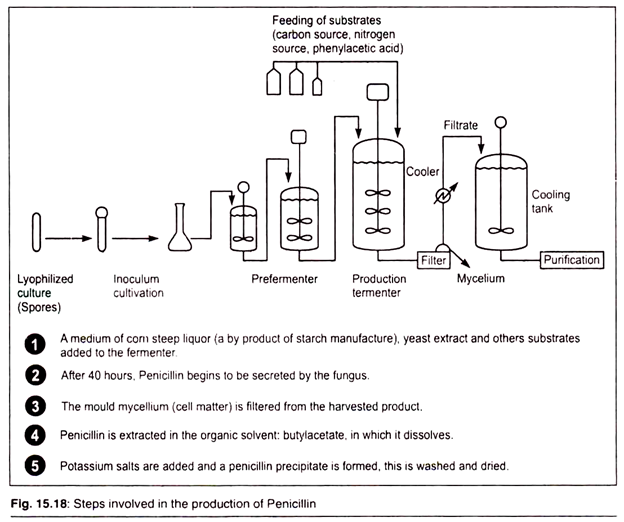
The Manufacturing Process:
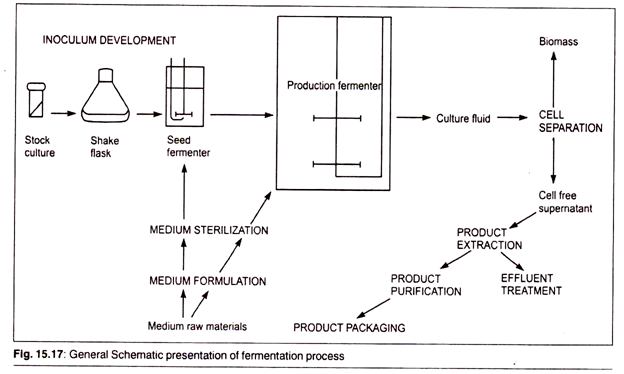
Although most antibiotics occur in nature, they are not normally available in the quantities necessary for large-scale production. For this reason, a fermentation process was developed. It involves isolating a desired microorganism, fuelling growth of the culture and refining and isolating the final antibiotic product. It is important that sterile conditions be maintained throughout the manufacturing process, because contamination by foreign microbes will ruin the fermentation.
A. Starting the Culture:
1. Before fermentation can begin, the desired antibiotic-producing organism must be isolated and its numbers must be increased by many times. To do this, a starter culture from a sample of previously isolated, cold-stored organisms is created in the lab. In order to grow the initial culture, a sample of the organism is transferred to an agar-containing plate.
The initial culture is then put into shake-flasks along with food and other nutrients necessary for growth. This creates a suspension, which can be transferred to seed tanks for further growth.
2. The seed tanks are steel tanks designed to provide an ideal environment for growing microorganisms. They are filled with all the things the specific microorganism would need to survive and thrive, including warm water and carbohydrate foods like lactose or glucose sugars. Additionally, they contain other necessary carbon sources, such as acetic acid, alcohols, or hydrocarbons, and nitrogen sources like ammonia salts.
Growth factors like vitamins, amino acids, and minor nutrients round out the composition of the seed tank contents. The seed tanks are equipped with mixers, which keep the growth medium moving, and a pump to deliver sterilized, filtered air. After about 24-28 hours, the material in the seed tanks is transferred to the primary fermentation tanks.
B. Fermentation:
The fermentation tank is essentially a larger version of the steel seed tank, which is able to hold about 30,000 gallons. It is filled with the same growth media found in the seed tank and also provides an environment inductive to growth. Here the microorganisms are allowed to grow and multiply. During this process, they excrete large quantities of the desired antibiotic.
The tanks are cooled to keep the temperature between 73-81°F (23-27.2°C). It is constantly agitated, and a continuous stream of sterilized air is pumped into it. For this reason, anti-foaming agents are periodically added. Since pH control is vital for optimal growth, acids or bases are added to the tank as necessary.
C. Isolation and Purification:
After three to five days, the maximum amount of antibiotic will have been produced and the isolation process can begin. Depending on the specific antibiotic produced, the fermentation broth is processed by various purification methods. For example, for antibiotic compounds that are water soluble, an ion-exchange method may be used for purification.
In this method, the compound is first separated from the waste organic materials in the broth and then sent through equipment, which separates the other water-soluble compounds from the desired one. To isolate an oil-soluble antibiotic such as penicillin, a solvent extraction method is used.
In this method, the broth is treated with organic solvents such as butyl acetate or methyl isobutyl ketone, which can specifically dissolve the antibiotic. The dissolved antibiotic is then recovered using various organic chemical means. At the end of this step, the manufacturer is typically left with a purified powdered form of the antibiotic, which can be further refined into different product types.
D. Refining:
1. Antibiotic products can take on many different forms. They can be sold in solutions for intravenous bags or syringes, in pill or gel capsule form, or they may be sold as powders, which are incorporated into topical ointments. Depending on the final form of the antibiotic, various refining steps may be taken after the initial isolation.
For intravenous bags, the crystalline antibiotic can be dissolved in a solution, put in the bag, which is then hermetically sealed. For gel capsules, the powdered antibiotic is physically filled into the bottom half of a capsule, then the top half is mechanically put in place. When used in topical ointments, the antibiotic is mixed into the ointment.
2. From this point, the antibiotic product is transported to the final packaging stations. Here, the products are stacked and put in boxes. They are loaded up on trucks and transported to various distributors, hospitals, and pharmacies. The entire process of fermentation, recovery, and processing can take anywhere from five to eight days.
E. Quality Control:
Quality control is of utmost importance in the production of antibiotics. Since it involves a fermentation process, steps must be taken to ensure that absolutely no contamination is introduced at any point during production. To this end, the medium and all of the processing equipment are thoroughly steam sterilized.
During manufacturing, the quality of all the compounds is checked on a regular basis. Of particular importance are frequent checks of the condition of the microorganism culture during fermentation. These are accomplished using various chromatography techniques. Also, various physical and chemical properties of the finished product are checked such as pH, melting point, and moisture content.
In the United States, antibiotic production is highly regulated by the Food and Drug Administration (FDA). Depending on the application and type of antibiotic, more or less testing must be completed. For example, the FDA requires that for certain antibiotics each batch must be checked by them for effectiveness and purity. Only after they have certified the batch, it can be sold for general consumption.
F. The Future:
Since the development of a new drug is a costly proposition, pharmaceutical companies have done very little research in the last decade. However, an alarming development has spurred a revived interest in the development of new antibiotics.
It turns out that some of the disease-causing bacteria have mutated and developed a resistance to many of the standard antibiotics. This could have grave consequences on the world’s public health unless new antibiotics are discovered or improvements are made on the ones that are available. This challenging problem will be the focus of research for many years to come.
G. Enzymes:
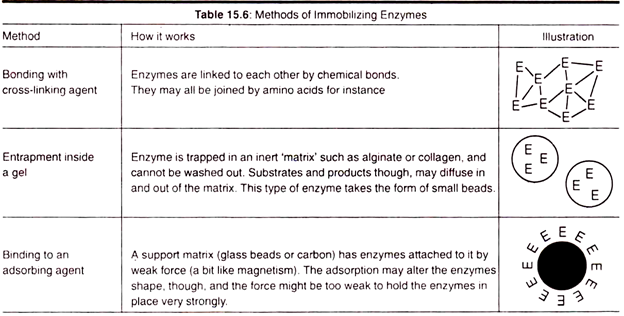
Micro-organisms can be used to produce enzymes that are useful in certain industrial applications. A more efficient method of using them is to isolate an enzyme and use it by itself. This is better because higher concentrations of enzyme can be obtained and only one reaction is taking place, whereas using whole micro-organisms would have numerous enzyme reactions and more products to process.
A further problem encountered is that enzymes are expensive to produce. This is solved by reusing the enzyme, this is made possible by immobilizing the enzymes, this way they do not contaminate the end products which can be reused. In the following table different methods of immobilization are discussed.
H. Monoclonal Antibodies (mAB):
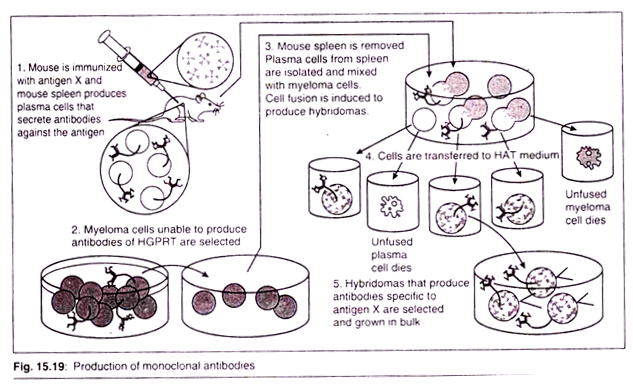
Hybridomas are cells that have been engineered to produce a desired antibody in large amounts, to produce monoclonal antibodies. Monoclonal antibodies can be produced in specialized cells through a technique now popularly known as hybridoma technology.
Hybridoma technology was discovered in 1975 by three scientists, Georges Kohler of West Germany and Cesar Milstein of Argentina and Niels Jerne of Denmark. They were awarded the 1984 Noble prize for physiology and medicine.
I. Methodology:
A hybridoma is produced by the injection of a specific antigen into a mouse, procuring the antigen-specific plasma cells (antibody-producing cell) from the mouse’s spleen and the subsequent fusion of this cell with a cancerous immune cell called a myeloma cell. The hybrid cell, which is thus produced, can be cloned to produce many identical daughter clones.
These daughter clones then secrete the immune cell product. Since these antibodies come from only one type of cell (the hybridoma cell) they are called monoclonal antibodies. The advantage of this process is that it can combine the qualities of the two different types of cells; the ability to grow continually, and to produce large amounts of pure antibody.
HAT medium (Hypoxanthine Aminopetrin Thymidine) is used for preparation of monoclonal antibodies. Laboratory animals (e.g.; mice) are first exposed to an antigen whose corresponding antibody we want to develop. Once splenocytes are isolated from the mammal, the B cells are fused with immortalized myeloma cells, which lack the HGPRT (hypoxanthine-guanine phosphoribosyltrans-ferase) gene, using polyethylene glycol or the Sendai virus.
Fused cells are incubated in the HAT (Hypoxanthine-Aminopetrin Thymidine) medium. Aminopetrin in the myeloma cells die, as they cannot produce nucleotides by the de novo, or salvage medium blocks the pathway that allows for nucleotide synthesis.
Hence, un-fused D cells die. Un-fused B cells die as they have a short life span. Only the B cell-myeloma hybrids survive, since the HGPRT gene coming from the B cells is functional. These cells produce antibodies (a property of B cells) and are immortal (a property of myeloma cells).
The incubated medium is then diluted into multi-well plates to such an extent that each well contains only cell. Then the supernatant in each well can be checked for desired anti body. Since the antibodies in a well are produced by the same B cell, they will be directed towards the same epitope, and are known as monoclonal antibodies.
Once a hybridoma colony is established, it will continually grow in culture medium like RPMI-1640 (with antibiotics and foetal bovine serum) and produce antibody.
The next stage is a rapid primary screening process, which identifies and selects only those hybridomas that produce antibodies of appropriate specificity. The hybridoma culture supernatant, secondary enzyme labelled conjugate, and chromogenic substrate, is then incubated, and the formation of a coloured product indicates a positive hybridoma.
Alternatively, immunocytochemical screening can also be used. Multi-well plates are used initially to grow the hybridomas and after selection, are changed to larger tissue culture flasks. This maintains the well-being of the hybridomas and provides enough cells for cryopreservation and supernatant for subsequent investigations.
The culture supernatant can yield 1 to 60 ug/ml of monoclonal antibody, which is maintained at 20°C or lower until required. By using culture supernatant or a purified immunoglobulin preparation, further analysis of a potential monoclonal antibody producing hybridoma can be made in terms of reactivity, specificity, and cross-reactivity.
J. Large-Scale Production of Monoclonal Antibodies (mAB):
Large scale production of monoclonal antibodies consider production scales of 0.1-10 g as small, 10-100 gas medium, and over 100 g as large. This is generally performed to produce monoclonal antibodies for three purposes: diagnosis, therapy, and research on and development of new therapeutic agents.
K. Amino Acid Production:
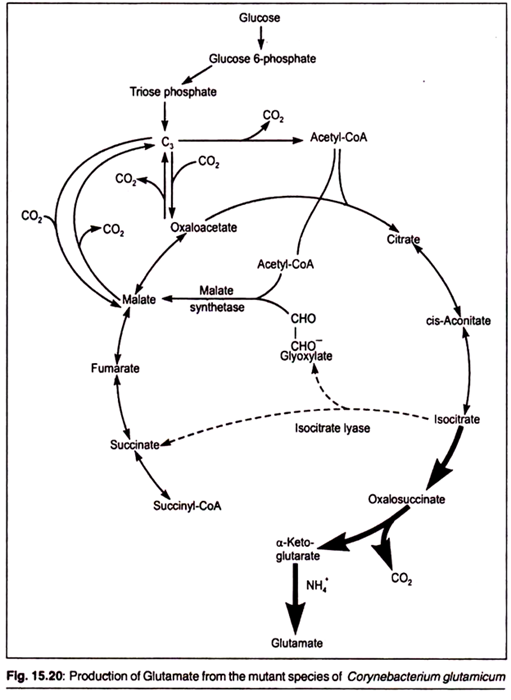
Amino acids such as lysine and glutamic acid have been used in the food industries for a long time as a nutritional supplement (in order to increase the nutritional value of the bread) and as flavor enhancing compound. Glutamic acid is produced in large quantities by using mutants of Corynebacteriumglutamicum that lack the ability to convert the TCA cycle intermediate alpha ketoglutarate to succinyl-CoA.
Lysine is also produced from the mutant bacterium Corynebacteriumglutamicum which blocks the synthesis of homoserine and accumulates lysine.
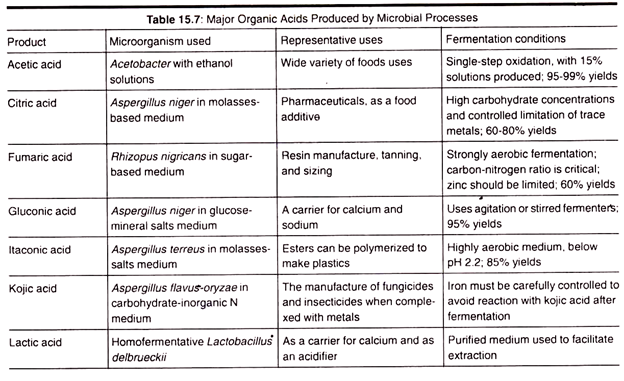
L. Organic Acid Production:
Organic acids such as Citric, acetic, lactic, fumaric, and gluconic acids are major products of microbial industry. Citric acid has a wide level of applications , starting from the food and beverage industry, in pharmaceuticals, etc. Aspergillusniger is used for the production of citric acid.
The fundamental idea behind the production of citric acid is to limit the availability of the amounts of trace metals such as manganese and iron to prevent the growth of Aspergillusniger at a specific point in the process of fermentation.
The medium for the citric acid fermentation contains high sugar concentrations (15 to 18%) and copper.The success of this fermentation depends widely on the regulation and functioning of the glycolytic pathway and the tricarboxylic acid cycle.
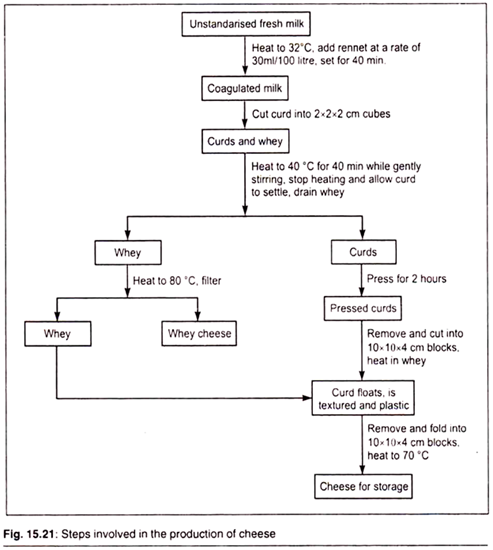
M. Cheese Production:
Cheese is produced from a lactic acid fermentation of milk, which results in coagulation of milk proteins and formation of a curd. Rennin is generally used to promote this curd formation. After the curd is formed, it is heated and pressed in order to remove the watery part of the milk called as whey.
The solid curd is then salted and kept aside for ripening. In the production of cheese, microorganisms are widely used. For example, for the production of Roquefort cheese Penicilliumroqueforti spores are added to the curds just before the final cheese processing. Also during the production of Camembert cheese, the cheese is inoculated with spores of Penicilliumcamemberti.
N. Production of Alcoholic Beverages:
Alcoholic beverages are basically produced from the fermentation of carbohydrates. The most widely consumed alcoholic beverages are wine and beer.
O. Production of Wine:
Wine is produced by the fermentation of grape juice. Folio wings are the basic six steps involved in the production of wine.
Step 1:
Viticulture:
As a known fact the flavour of the wine is dependent on the kind of grapes which are used. The variety of grapes depends on the place where they are grown, the climate of the place where they are grown, the drainage system around the winery, the quality of the soil, humidity content of the region and even the exposure levels to the sun.
Other than these factors, one of the most vital points which is an important determinant in the making of wine is the techniques used to make the wine. In fact, the different wineries formulate a customized wine making procedure according to the needs of their winery, so that they can bring out the best flavours of their grapes.
Step 2:
Harvesting:
The next step in the wine-making process is the harvesting of the finely cultivated grapes. When the grapes are ripped and harvested it is very important that the timing of ripping the grapes is apt and the exact timing can be ascertained through experience.
The timing of the ripping the grapes should be such that the grapes have the apt combination of sugar, acid and moisture. The harvesting of the grapes can be either done manually or mechanically, however majority of the grape wineries reap it manually.
Step 3:
Crushing:
The next step after the grapes have been harvested is to crush and press so as to have their inherent flavours in the form of liquid. As the fruits Eire crushed the grapes withdraw their moisture content and the sugars.
Many wineries make the usage of specialized machines for the process of crushing and pressing the grapes. The resultant liquid which is formed after crushing and pressing the wines is referred to as must, at this stage the wine will either turn into red colour or white colour depending on the preference of the wine-maker.
The wines become red when they are simply left after, they are crushed because in this stage juices from the skin and the flavours are ripped off, thus when you leave the must for a certain period of time, the wine become red wine, and in case you immediately crush the wine after pressing and segregate the skin, then the wine becomes white wine.
Step 4:
Fermentation:
After the grapes are being crushed and pressed, the fermentation takes place. Since the grapes have good quantity of sugar and moisture they easily get fermented with the reaction of wild yeast. Fermentation takes place in almost 10 to 30 days; however, this depends on the quality of grapes and the climate.
Step 5:
Clarifying the Solution:
The next step in wine making is clarifying the solution. It is also referred to as stabilization.
Step 6:
Bottling:
The final stage is to transfer the clarified solution in the wooden barrels or bottles.
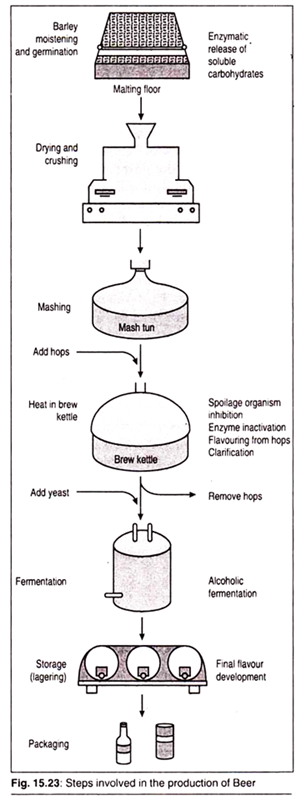
Production of Beer:
Step 1:
Extraction:
Beer-makers use grains or extracts to begin the mash, the base that will define the taste of the finished beer. Grains of barley or wheat are pre- soaked to begin the release of enzymes to produce maltose, a sugar. The brewer crushes a mixture of malted and un-malted grains and then heats them in water.
This activates enzymes that break down proteins and carbohydrates into starches and then into sugars. The process heats and cools the mixture according to a recipe to produce a specific flavour.
When finished, the liquid is drained through the sediment and the grains are dried in a kiln. The dried grain is soaked in warm water and drained again to extract more sugars from the mash. A final rinse with fresh hot water pulls out the last of the sugars.
The three extractions produce a liquid called “wort,” which is brought to the boil to kill any harmful bacteria and concentrate the extract. Hops, a cone-like bloom, contains oils and resins that offset the sweetness of the sugars. It may be added to the wort during the “boil” to “bitterize” the mash before fermentation or it may be added at the end of fermentation, depending on the recipe and brewer.
Each mix of grain and hops produce different types and amounts of sugars to be available for fermentation, the next process in brewing.
Step 2:
Fermentation:
Fermentation is a chemical process. The brewer adds yeast (Saccharomyces cerevisiae), a type of fungus that metabolizes sugars in the absence of oxygen, to the aerated wort. As fermentation begins, a foamy coat of “krausen” grows on the surface of the wort and the chemical reaction produces carbon dioxide and alcohol.
A valve on top of the sealed fermentation chamber vents the carbon dioxide as the fermentation progresses.
The chemical reaction also produces esters and phenols that contribute to the character of the beer’s flavour as well as chlorine and fatty acids. Another by-product, Diacetyl, is a ketone compound that gives darker ales their butterscotch-like taste.
Lactic acid is often added to wheat wort to add tartness. Temperature is used to control the fermentation process, but eventually it will stop because the sugars have been consumed and most of the yeasts have died.
Step 3:
The Finish:
The final process of brewing is bottling but many brewers insert intermediate steps in this finishing process. The fermented wort must be separated from the foamy krausen and solid remnants of hops, dead yeast and other by-products that may coat the sides of the chamber, often by draining from the bottom or siphoning.
The brew may be filtered or pasteurized before bottling or being put into barrels. As beer is bottled or barrelled, a bit of sugar—white sucrose, brown or molasses—is added to it to encourage the remaining live yeast to ferment and vent carbon dioxide, which will stay in the sealed container as carbonation.