In this essay we will learn about Growth of Plants. After reading this essay you will learn about: 1. Introduction to Growth of Plants 2. Stages in Growth of Plant 3. Measurement 4. Effects of Light 5. Growth Regulators 6. Other Growth-Regulating Substances 7. Synthetic Growth Regulators.
Contents:
- Essay on the Introduction to Growth of Plants
- Essay on the Stages in Growth of Plants
- Essay on the Measurement in Growth of Plants
- Essay on the Effects of Light in Growth of Plants
- Essay on Growth Regulators in Plants
- Essay on Other Growth-Regulating Substances from Plants
- Essay on the Synthetic Growth Regulators in Plants
Contents
- Essay # 1. Introduction to Growth of Plants:
- Essay # 2. Stages of Growth in Plants:
- Essay # 3. Measurement of Growth in Plants:
- Essay # 4. Effects of Light on Growth in Plants:
- Essay # 5. Growth Regulators in Plants:
- Essay # 6. Other Growth-Regulating Substances from Plants:
- Essay # 7. Synthetic Growth Regulators in Plants:
- Essay # 7. Mechanism of Action of Growth Regulators in Plants:
Essay # 1. Introduction to Growth of Plants:
One of the most self-evident of all natural phenomena is the continuous increase in size of plants and production of new organs at least during some stage of their life histories. The term growth is popularly employed to designate this complex of processes, which depends on many other physiological processes.
It is impossible to understand plant growth without a sound knowledge of the basic facts of absorption, transport of materials such as minerals to the place of synthesis and metabolism itself. Besides these processes other factors specific to growth and development such as hormones must be mentioned. The advances made in this field during the previous 30 or 40 years have really been tremendous.
Growth may be defined as a permanent and irreversible change in any dimension of an organism.
The dry matter which is incorporated into the structure of both protoplasm and cell walls during growth comes almost entirely from the foods synthesised by plants themselves.
In the synthesis of new protoplasm, i.e., in the production of new cells, the foods which are assimilated are largely proteins, for protoplasm, as we know, is largely proteinaceous. Foods which are assimilated in the formation of cell walls are mostly carbohydrates, e.g., cellulose and pectins.
As a result of assimilation, a growing region, such as stem tip or root tip increases in dry weight during growth, i.e., new cells are continuously being formed from pre-existing cells.
The mere increase in size of a plant or plant part is not necessarily, the result of growth. We know that dry seeds when soaked in water, imbibe water and certainly increase in volume and in weight. But that cannot be considered as growth for removal of water will also reduce the volume of the seeds to original volume and size and there is also no increase in dry weight.
We know that growth of plant occurs in regions of growing points known as meristems.
Three main changes occur in meristems:
Cell division, cell enlargement and cell differentiation.
The growth of a plant may be due either to cell division or to cell enlargement.
This is possible only because the two daughter cells produced, enlarge to the approximately same size of the mother cell, before they divide again. Thus the growth of a plant or plant organ is essentially an increase in size due to an enlargement of cells and this cell enlargement must be irreversible and not simply a reversible increase due to normal increase in hydrostatic pressure, caused by turgour.
In classical concept of cell growth, the causative force is turgour pressure. Yet it is a fact that mature cells have higher turgour pressure than younger meristematic cells. The mature cells, however, have more rigid cell walls but the growing cells stretch more readily.
Consequently a small turgour pressure is sufficient to stretch the walls of a young growing cell which may not be sufficient to stretch the wall of a more mature cell. As water enters the cell during cell enlargement, solutes also are absorbed.
In this way, the cell maintains a high osmotic pressure of the cell sap, and conditions for the development of sufficiently high turgour pressure to stretch the cell walls, are well maintained.
Besides water and solute absorption other changes also occur during cell growth. New cell wall materials are laid down by intussusception, i.e., insertion of new molecules of cellulose on the old wall particles that become softened to permit the stretching the wall undergoes during enlargement.
Thickening may also take place by opposition, i.e., deposition of new cell wall matter on the inner surface of the wall during the maturation phase of the cell.
The meristematic cell is filled with cytoplasm and has only a few very small vacuoles. As the cell enlarges, the vacuoles increase in number and by coalescing with each other, occupy a much larger proportion of the cell and the cytoplasm as a result becomes a thin layer, lining the large central vacuole.
Thus growth of a cell can be said to involve the following primary processes:
1. Absorption of water,
2. Absorption of solutes for maintaining higher turgour pressure of the cells,
3. Deposition of new cell wall material on the stretched cell walls,
4. Formation of new protoplasm by synthesis of proteins, lipids, etc.
Growth is therefore a very complex process involving nearly all the other physiological processes known to occur in the plant.
And it requires expenditure of large quantities of energy since absorption of solutes is probably active and not passive (osmotic), completely depending upon energy released in respiration. The synthesis of cell wall materials as well as the synthesis of protoplasmic substances is also endergonic processes.
Essay # 2. Stages of Growth in Plants:
The growth of an entire plant or only of a plant organ characteristically passes through stages represented by a S-shaped curve. The time during which this occurs has been called grand, period of growth (Fig. 736). It has been assumed that the rate of production of new materials in growth is proportional to the size of the plant. Thus the growth of an annual plant probably follows a compound interest law.
The difference between simple interest and compound interest is that when increase takes place at a rate proportional to the initial quantity, it is following a simple interest formula. When the increase occurs at a rate proportional to the new quantity at each stage, it is the compound interest formula.
For example, Rs. 100.00 at 5% interest becomes naturally Rs. 110-00 after two years. But in compound interest law Rs. 100-00 at the same 5% interest becomes 105 after one year, but at the end of second year where as in simple interest the total becomes 100+5+5, i.e., 110, in compound interest it is 105+5% of 105 and so on.
Blackman applied the compound interest formula to explain growth:
W1 = W0ert or in logarithmic form, loge (W1/W0) = rt or 2.3026 log [W1/W0) = rt
where W1 = final dry weight attained by an annual plant.
W0 = initial dry weight, e=base of natural logarithm)
r = rate of increase in dry weight per unit time, and f=time interval.
Thus if a plant has doubled itself as regards increase in dry weight in, say, 15 days,
i.e.,
W1/W0 = 2. Since loge2 = 0.69315
the rate of increase in dry weight is:
r = loge (W1/W0) ÷ 15 = (loge2) ÷ 15 = 0.693154÷15 = If the period of doubling were 10 days, the rate would be 6.93 per cent, per day.
It has been assumed that growth is dependent on chemical transformations of reserve substances into new living protoplasm. The new protoplasm as soon as it is formed begins to participate in the mechanism of transformation by apparently catalysing its own synthesis (autocatalytic reaction). Following this principle, the theoretical curve obtained resembles the typical S-shaped curve (Fig. 737).
Essay # 3. Measurement of Growth in Plants:
Rate of growth can be measured by various ways.
Any one of the following measures can be suitably applied in determining the growth rate and total growth of the individual organs of a plant or the plant taken as a whole:
(a) Increase in weight,
(b) Increase in volume,
(c) Increase in height,
(d) Increase in the number of cells produced and so on.
Rate of growth can be conveniently measured by standard instruments like the simple arc indicator or by more complex auxanometers. Auxanometers give a more detailed idea of growth rates both during day and night. The revolving drum, with the pasted smoked paper on which the end of a pointer needle is attached, can be easily made to record the rate of a growth in a particular period, say, 72 hours (Fig. 738).
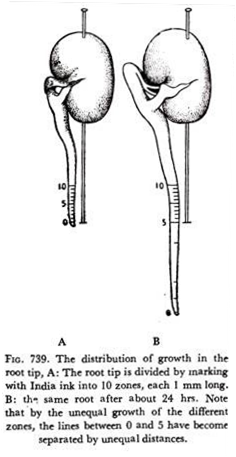
In the apical meristem of stems and roots, the rate of growth is not uniform throughout its length. If the apical portion of the radicle of a germinating seeds is marked into equal divisions it is found that the growth rate is maximum not at the very apex, but at more basal regions, a few mm from the apex.
If the apical root meristem is divided into ten equal divisions from the tip as in Fig. 739, it is seen that the portion of tissues between divisions two and four has increased most. Evidently the growth of the tissue in the more basal region is controlled and maintained by some sort of stimulus which might be originating at the very tip which is not actually the growing region.
The growth of the individual leaf is not also uniform throughout its entire surface. This can be demonstrated with the help of a space marker disc (Fig. 740). Two leaves, one sessile and the other with petiole, are marked near the tip, the middle and at the base by the space maker disc.
After a few days, the markings left on the leaf may be found to be wider apart in the tips of the leaf with petiole, and around the base of a leaf without petiole. The markings in the middle portion in both types of leaves remain more or less uniform.
Essay # 4. Effects of Light on Growth in Plants:
Of the climatic factors—temperature and light are the most important. Growth of higher plants occurs in the range of temperature of about 0.35°C. Within this range, raising of temperature by 10°C, increases the rate of growth 2—3 times (Q16=2 or 3) if other factors are not limiting.
There are three, certainly not very sharp, temperature zones, known as the cardinal points for growth—the minimum, optimum and maximum (Fig. 741).
Effects of light on growth are at least four-fold., each aspect having its own particular type of response from the plant. They are intensity of light, quality of light, duration of light and the direction from which the incident rays fall on a plant.
The following characteristic responses of the plant to the various aspects of light on growth can be recognised:
Intensity of Light:
The effect of high intensity of light on growth is, in general, to retard the growth process (except for photosynthetic function).
Quality of Light:
Shorter rays beyond the violet and longer rays beyond far red are detrimental to growth. Red and far red light seem to be the most active for regulation of growth. The spectral quality of light is very important, for the plant contains perceptive pigments responsive only to light of particular wave-lengths.
Duration of Light:
Effect is restricted to certain physiological processes the most remarkable of which is photoperiodisrn.
Direction of Light:
Shoots, some roots and leaves and also other organs usually show different orientations to the direction of the incident light—the phenomenon of phototrophic curvature.
Though the growth of higher plants eventually depends on photosynthesis, nevertheless light is not essential for the growth process itself, so long as sufficient quantities of organic substances are available. But the kind of growth is different when light is absent.
In the dark, higher plants show a weak, excessively elongated growth in case of most dicotyledons but the leaves are underdeveloped. Little tissue differentiation, however, occurs in the dark, the tissue remaining mostly parenchymal.
Other non-green plants also show a similar stretching in darkness. Monocotyledons as a rule show excessive elongation of the first internode only in darkness and may also then show excessive leaf development. Light actually retards all this excessive growth in the dark.
An extreme case is the dwarfing of alpine plants (plants growing in high altitude, above 3,600 metres in tropics) by intense light to which they are subjected to.
The light in the alpine region is very rich in violet and ultra-violet radiations and it seems that in general, light of smaller wave-lengths as also light waves longer than far red, have specific inhibitory effect on vegetative growth.
Among the edaphic factors, available water in the soil must take a pre-eminent place since all growth, as we know, depends on the development of a considerable turgour pressure of the cells.
Consequently a deficiency of water supply to the regions of meristematic activity will, of course, retard or completely stop growth. Thus, all factors ‘ influencing or facilitating absorption of water will naturally influence growth, if other factors are not limiting.
Some of the above factors may completely stop growth. This is most commonly the case in nature when the temperature is below the minimum for growth (e.g., in severe winter) or when its water content is too low (e.g., seeds). In this stage of suspended growth, the plant is said to be dormant.
Then, even if environmental conditions are favourable for growth, the plant may still fail to grow because of lack or scarcity of certain internal factors. In this state, it is said to be in a rest period. Newly harvested potato tubers fail to show any growth though the temperature, water and other environmental conditions are optimum for the growth of older potato tubers.
This rest period can be broken by artificial means such as treating with hormones or some chemicals or other methods such as removal of the terminal bud (the lateral buds are thereby forced). Other treatments include warm baths and also subjecting the organ at rest period, to alternate freezing and thawing and scarification.
Essay # 5. Growth Regulators in Plants:
Among substances which markedly influence reactions and metabolism of the animal body are hormones which are internally secreted by glands, (e.g., adrenaline, insulin, thyroxine, etc.) and vitamins. Convincing evidence has accumulated that similar hormone like substances also occur in plants.
These compounds are called growth substances, hormones, growth hormones or phytohormones. It is characteristic of both plant and animal hormones that they usually exert their physiological effects while present in minute concentrations.
Such concentrations are difficult to measure by conventional chemical methods but are bio-assayed using plant materials-as test objects, since plant organs exhibit detectable response in presence of low concentrations of the hormones and the response, within limits—has a quantitative relationship with concentration.
Plant hormones, however, as far as is known until now, are not produced in any glands. They may be produced in growing regions like shoot tips or root tips or in leaves and flowers and growing fruit tissues. Like animal hormones they are usually active in target organs some distance away from the site of synthesis.
The physiological effects are exhibited throughout growth and developmental phases—from germination to senescence and thus such hormones should not be referred to as “growth hormones” or “growth substances“—because growth is only one of many physiological responses they evoke.
The same hormone may be active in different target organs, but the optimum concentrations for the different responses they evoke, vary. Many animal hormones are polypeptide or steroidal in nature. While no plant protein or polypeptide has so far been found to possess hormonal properties, some plant phytosterols and terpenoids —di and tri— have been found to be biologically active.
They have not yet been accepted as “hormones” although their regulatory role has been recognised. Plant hormones have considerable similarities with animal hormones—in their basic pattern of mode of action—particularly in relation to the long term effects. The molecules of plant hormones are much smaller, the smallest being ethylene—C2H4.
Plant hormones usually exhibit two phase concentration curves, i.e., low concentrations may evoke promotive responses, whereas at higher concentrations the degree of promotion may be reduced or the effect may be inhibitory.
They interact with some site (s) in the cell, which apparently become saturated at relatively low concentrations, beyond which there is a competition among the molecules, due to which proper attachment with the site does not occur and the response is decreased or almost non-existent.
Several groups of plant hormones are now known. These are classified according to their physiological activity.
The major classes are:
(a) auxins,
(b) cytokinins,
(c) gibberellins,
(d) ethylene and
(e) abscisic acid.
In addition to these there are the leucoanthocyanins, brassicins, traumatic acid, helminthosporol etc. Phenolics, coumarins and many tetra- and pentacylic tri-terpenoids have inhibitory properties—and they are referred to as “inhibitors“—although at very low concentrations some of them may also promote growth.
Many synthetic compounds have been prepared which stimulate the naturally occurring hormones in their physiological activity; some of them also have some structural similarity with the natural hormones. The synthetic substances because they can be produced by synthetic means in large quantities—are much cheaper and are thus used in agriculture and horticulture.
But they should not be referred to as “hormones“, because they do not occur in nature. The natural and synthetic growth regulatory substances together are termed “growth regulators“; they include both promoters and inhibitors.
We shall now discuss very briefly the occurrence, properties and physiological activity of the more important classes of growth regulators.
A. Auxins:
When Avena coleoptile tips are exposed to light coming from one side, the coleoptile bends towards the direction of light. If a coleoptile tip is decapitated and removed by a cut made several mm below the apex, the rate of growth of the stump is immediately retarded. If, however, the tip is replaced on the stump, growth is resumed.
Such experiments indicate that the growth of the coleoptile which occurs in the more basal regions is maintained only under the influence of some sort of stimulus originating at the tip and which is transmitted from apex to base through the coleoptile.
If the cut-off tips of the oat coleoptiles are placed on a thin layer of agar, after an hour the cut tips are removed and the agar cut into a number of equal sized small blocks, then each one of these blocks, when placed upon the stump of a decapitated coleoptile can accelerate the growth of the stump almost as much as an intact coleoptile.
It seems evident from these results that some substance or substances was transported out of the tip into the agar block and subsequently out of the block into the decapitated stump, when it was translocated downwards to the elongating region (region of response) of the coleoptile. The substances which induce such responses are now classed as auxins. Auxins are apparently universally present.
They are non-specific in their action, i.e., the same auxin or auxins, chemically speaking, which influences growth phenomenon in one species apparently also influence the same phenomenon in all pieces. The auxins seem to be used up during the physiological process they influence and thus lack this and other characteristics of enzymes. The tern: auxin was first coined in about 1905.
Auxins are known to be of widespread distribution in plants. They occur in very small quantities and their detection by standard chemical methods is usually difficult and often impossible. With the discovery of a new, easily handled technique early in the last decade—partition chromatography on paper as also thin layer chromatography—precise identification of all naturally occurring auxins has been possible.
While in the past, auxins were estimated by curvatures such as those in the Avena test or in pea test, now any critical auxin analysis of plant extracts must involve separation and identification of auxins by the chromatographic techniques before estimation because there are several auxins and auxin precursors as also some non-auxinic substances which may promote or inhibit growth.
The chronological sequence of the development of our early knowledge of auxins in plants is illustrated in the following pages:
Indole Auxins:
The term auxin is defined here to include only growth promoting substances active in the Avena or similar bioassays. However, in addition to this, auxin also promotes combium activity, xylem formation, protoplasmic streaming and root initiation, inhibits bud growth, helps formation of female flowers, and stimulates the setting and growth of fruits and causes abscision depending on concentration.
The principal naturally occurring auxins in plants that have been definitely identified, isolated, purified and their molecular configuration determined are all indole derivatives as mentioned below.
(i) Indoleacetic acid (IAA—mol. wt. 175)
(ii) Indoleacetonitril (IAN—mol. wt. 156)
(iii) Indoleacetaldehyde (IAAld—mol. wt. 159).
(iv) Ethylindoleacetate (mol. wt. 203)
(v) Indole-3-pyruvic acid (IPyA—mol. wt. 203)
(vi) Indole-3-ethonol (IEtOH—mol. wt. 161)
There is a controversy regarding whether ethylindoleacetate occurs naturally or is produced during extraction as an artifact. Occurrence of indoleacetamide is also not conclusively established.
The presence of indole ethanol in plants also is questionable.
Non-indole compounds, e.g., some fatty acids may also possess auxin-like properties. But they have not yet been properly characterised. Many synthetic compounds resembling auxins in their biological properties are known; they usually have some structural similarity to indole acetic acid.
Auxins are present in tissues in very low cocentrations and are bio-assayed by the coleoptile curvature or straight growth test, internode elongation test, slit pea test, tomato ovary test, leaf epinasty test etc.
Although indoleacetic acid was known to chemistry since 1885, its biological activity was not suspected and it was recognised as a plant growth substance only in 1934 when it was identified and extracted from urine and termed, heteroauxin.
Even then it was by no means clear that IAA was the native auxin of higher plants; on the contrary it was generally held that ‘auxin a and b’ were the primary auxins of higher plants while IAA was a product of fungi and bacteria.
By the end of 1950 however, it was becoming clear that indoleacetic acid is the principal naturally occurring auxin in all higher plants. The previously known ‘auxin a’ and ‘ auxin b’ isolated from plant tissues by Kogl (1934) are now considered to be only of theoretical importance for, although they have been claimed to be isolated back in 1934, since then, to say the least, they have proved themselves very elusive indeed.
When applied uniformly to decapitated coleoptiles,’ IAA causes increased growth; when applied to one side, it causes curvature towards the other side. The greatest puzzle of all seems to be the mechanism whereby traces of auxin such as a relatively simple organic acid, like IAA and its derivatives can so dramatically bring about growth or cellular enlargement.
The position of the side-chain in the ring structure in indole-3-acetic acid appears to be highly specific for activity, since 1-, 2-, and 4-indole acetic acids are only very slightly active in bioassay. Substitution with halogens, like fluorine and chlorine can result, however, in very active derivatives, e.g., the 4-chloro and 6-chloro compounds.
The replacement of an aromatic CH by N can give, 1-aza (indole-3) acetic acid (Fig. 743, VII) which is less active in the Avena test or indazole-3-acetic acid (Fig. 743, VIII) which has activity equal to IAA.
IAN may be converted into either IAC (indole-3-carboxylic acid) or IAA. When the imino group (NH) in IAA is replaced by a sulphur atom (Fig. 739, IX), the resulting compound, thianaphthen-3-indole acetic acid is quite active in various bioassay tests (Allsopp, 1965).
Indole propionic acid (IPA) is less active than IAA, but indole butyric acid (IBA) is morel active than IPA; in some tests as in the case of rooting of cuttings, IBA is even as or more active than IAA. Side chains having even number of C-atoms are more active than those having an odd number.
It is usually believed that the side chain is oxidised to IAA before the compound shows a biological activity. The chemical structure of some synthetic auxins is given in fig 744.
Indoleacetonitrile (IAN), a nitrile derivative of indoleacetic acid, is a neutral substance which has been obtained in crystalline form from a number of plant materials. It is believed that the nitrile, IAN, as such is inactive and has to be converted to IAA in order to be active. Upon alkaline hydrolysis the nitrile yields indoleacetic acid.
It has been found that IAN promotes growth of only those plant organs, e.g., Avena coleoptile, bean seeds, tomato ovary etc., which possess the enzyme indole aceto nitrilase while tissues, like pea roots which cannot convert IAN to IAA are unreactive to IAN. In higher concentrations IAN is inhibitory like IAA.
An ethyl ester of IAA—ethylindoleacetate, C8H6N.CHaCOOC2H6—has also been isolated from plant tissues. Both IAN and this ester seem to be more active than IAA, in those tissues which are capable of converting them into IAA.
This may be due to the better penetration of these neutral substances than the acid itself. The aldehyde derivative, indoleacetaldehyde, CgH6N.CH2CHO, the nitrile, IAN and the ethyl ester all three exist in nature as auxin precursors and all of which can be converted into IAA.
In addition to acting as an auxin precursor, IAN is also known to be an activator or booster of IAA-responses. For although IAN is unable to bring about a response in pea test curvature even at 20 p. p.m., its presence in the medium, boosts the response of 0.1 p. p.m. IAA from 2° to nearly 60° curvature.
The work, of Fawcett, however, indicates that the activities of IAN are not always governed by its conversion to IAA.
Indole-3-pyruvic acid (IPyA) has been isolated from corn.
Phototrophic curvature is due to higher concentration of auxin on the side of the coleoptile farther from light. This may be due to either a transverse translocation of auxin from the illuminated side to the darker side or to a photochemical destruction of auxin (destruction activated by light absorbed by β-carotene or riboflavin) of the lighted side. Geotropism can also be explained in terms of auxin distribution.
Evidence exists that in addition to free auxins, which are present in all growing regions such as germinating seeds, in seeds there are also stored precursors of auxin in which auxin is bound to proteins, carbohydrates, amino acids, ascorbic acid (ascorbi- gen) etc. which change into IAA proper when seeds absorb water.
This change may occur in light- 01 in the dark. The precursors give rise to auxin predominantly in the apical regions.
The cyclic amino acid, tryptophan is regarded as a piecursor of IAA. When proteins are hydrolysed, tryptophan is liberated and this tryptophan undergoes oxidative deamination and subsequent decarboxylation to yield indoleacetaldehyde which is oxidised to the free auxin (IAA). The importance of Zn and cobalt ions in auxin formation is recognised.
There is a difference in sensitivity of roots and stems to the concentration of auxin. The same concentration (e.g., 10-6M) which causes increased growth of the stem cells may inhibit growth of roots cells. Thus cells may be either stimulated or inhibited, depending on the concentration of auxins.
This only applies to increase in elongation. On the other hand for root initiation or new root formation, a relatively higher concentration of auxin is necessary, which concentration, however, completely inhibits shoot formation or lateral bud growth.
This dual effect of auxin provides the plant with a switch mechanism for turning the growth process on or off. We have seen that when terminal buds grow, the growth of the lateral buds is inhibited. If the terminal bud is removed, the growth of the lateral buds may be stimulated which otherwise would have remained checked.
The terminal bud perhaps is able to prevent the growth of the lateral buds (apical dominance) by liberating excess auxin to the lateral buds, thereby preventing, (i.e., excess concentration of auxin) the growth of the lateral buds.
Thus, the lateral buds may be kept in the dormant state. When the terminal bud is removed, the resumption of growth of the dormant lateral buds may be due to lowering of the concentration of auxin to an effective level, which would now stimulate growth.
In potato tubers and many seeds, auxins are present in growth-stimulating quantity but the tubers and seeds are kept dormant (gibberellins can break this dormancy of potato tubers which action is contrary to that caused by IAA) by the presence of inhibitors which prevents the proper functioning of auxin.
The effects of auxins on growth result from an increased or decreased cell enlargement at low or high concentrations respectively. Auxins also affect cell division; callus tissues may be formed when IAA is applied to a cut stump. Renewed activity of cambium and apical meristems seem to be dependent on auxin concentration.
We know that the transport of auxin is usually polar from morphological apex to base. More information is, however, required as to the route and mechanism of transport of these comparatively smaller molecules of IAA throughout the body of the plant.
During recent years, apparently contradictory effects of auxin, in the phenomena of fruit-set and fruit-drop, have brought out one rather general principle, namely production of auxin is a transient activity of the developing tissue which is soon given up and taken over in time by other tissues.
It seems probable that some kind of balance is involved between IAA and gibberellin (see later) and that buds or fruits continue to develop only when the balance is maintained, while excess of either one, inhibits.
Auxins apparently control the growth of only those organisms that show marked cell enlargement, the higher plants. They have no unequivocal effect on the growth of microorganisms or fungi. No growth response is induced by plant auxins in organisms without cellulose cell wall, e.g., animals.
It has been amply proved that the cell enlargement induced by auxin is not due to any increased turgour pressure but perhaps due to the increase in plasticity of the cell wall brought about by auxins and which is certainly in some way linked with respiration, for it is known that auxins markedly affect respiration rates in plants.
Thus, due to the influence of auxin, plasticity of the cell wall increases, i.e., cell walls soften, thereby allowing osmotic pressure inside the cell to stretch them further as more water enters the cell.
During this process, new cell wall material is constantly laid down and relatively less cellulose but more pectin material, is initially laid down. IAA thus causes elongation as well as enlargement of individual cells by reducing the cell wall-pressure, thereby considerably increasing water uptake or suction pressure of the cells.
IAA certainly induces softening of the cell walls. A good deal of efforts has already naturally been devoted to an analysis of cell wall metabolism in connection with auxin actions. It now seems clear, however, that these changes can only be a secondary sequence of events occurring elsewhere in the cell, that is, the changes must be of more fundamental nature.
How does an auxin produce such diverse physiological responses, as mentioned earlier, in plants? We do not as yet know the initial reaction which causes auxin to set off a chain of events which ultimately expresses itself as a physiological response. Very likely it is a physico-chemical event concerning the whole cell, which changes the enzyme matrix or pattern inside the cells.
Synthetic Auxins:
Several small molecules have been snthesized (Fig. 744), which exhibit biological properties characteristic of IAA, though not in all respects. They are usually derivatives of benzoic acid, phenoxy acetic acid and naphthalene acetic acid.
Some of the potent compounds are: 2, 6-dichloro—and 2,3,5—triodo benzoic acids (TIBA), 2,4— dichloro (2,4—D)—and 2,4,5,-trichloro phenoxy acetic acid (2,4,5—T), α-naphthelene acetic acid (NAA), β-naphthoxy acetic acid etc. Phenyl acetic acid is a weak auxin.
An acidic side chain, unsaturation in the ring and an unfilled ortho-position usually characterise many active molecules, but no general rule can be framed. Some thiocarbamates which do not satisfy most of the requirements are quite active.
The D-forms are usually more active than the L-form; cu-cinnamic acid is an auxin, whereas trans-cinnamic acid behaves as an antiauxin. The three-dimensional structure of the molecule and the spatial relationship between the side chain and the aromatic ring, when present, determine biological activity.
These compounds which give positive tests in Avena coleoptile curvature bioassay are now proving to be of immense practical value to the agriculturist and horticulturist for such uses as promotion of flowering, selective weed control, rooting of cuttings, prolonging and breaking of dormancy of cuttings as also of seeds and buds, production of seedless parthenocarpic fruits, thinning of blossoms, prevention of premature, pre- harvest fruit-drop, etc.
Glucobrassicin:
Although glucobrassicin has been reported to be inactive in the straight-growth test, nevertheless it was found highly active in the Avena curvature bioassay. Glucobrassicin was first of all isolated from cabbage leaves. These compounds are members of the so-called mustard oil glucosides of glucosinolates.
B. Cytokinin:
The isolation and identification of kinetin, (6-furfurylaminoprine) from nucleic acid preparations, was first reported by Miller (1956). In extracts of rapidly growing tissues, such as immature and developing fruits, evidence of kinetin-like activity was obtained by numerous investigators.
Assay technique for this type of activity showed these substances effect of inducing rapid cell division in undifferentiated plant tissues, tissue cultures or callus, with IAA being required as a synergistic cofactor, in most systems.
Unlike auxins cytokinins promote bud growth. They also defer senescence and help in the retention of chlorophyll. Cytokinins are usually assayed by their effects on callus growth (soybean, tobacco etc.), expansion of cotyledons (cucumber etc.) and retention of chlorophyll in ageing leaf discs.
The term cytokinin has been accepted practically universally, as a generic name for substances which promote cell division and exert other growth regulatory actions in the same manner as kinetin.
Two such substances which have been isolated in crystalline form from Zea mays immature kernels by Letham (1964) and Koshimizu (1967) have been named zeatin and dihydrozeatin.
The identification of cytokinins as constituents of t-RNA, suggests that cytokinin moieties in t-RNA may have important role in protein synthesis. Isopentenyl adenine is highly active.
Zeatin riboside (Letham, 1966) and zeatin ribotide (Letham 1966, Miller, 1967) also occur naturally in plants; zeatin and zeatin riboside have also been isolated from mycorrhizal fungus (Miller, 1967). A cw-ribosyl zeatin and a ms-ribosyl zeatin have also been extracted from plant tissue.
Diphenyl urea and several of its derivatives have cytokinin activity. Several synthetic cytokinins are available. These include benzyl adenine and benzimidazole. Adenine also possess some cytekinin activity.
The chemical structures of some cytokinins are given in Fig. 745.
C. Gibberellins:
The field of gibberellins may be said to be truly a product of both East and West.
This strikingly potent and unusual group of growth substances—the Gibberellins —have been attracting the attention of botanists all over the world since the end of World War II. These compounds stimulate the growth of many plants, promote flowering in some cases and cause a variety of other interesting morphological and physiological responses. 
They have been known to the Japanese agriculturists during the past 50 years [a farmer in Japan, as far back as 1929, had actually described the symptoms of a disease of rice plants (locally known as ‘bakanae‘ meaning foolish) in which there was appearance of very tall and thin plants, sometimes pale, with longer and narrower leaves markedly overgrowing their unaffected neighbours] that a rice-disease causing ascomycetous fungus—Gibberella fujikuroi (the perfect form, occurring only occasionally; the imperfect form is Fusarium moniliforme) was responsible for the production of these substances.
Gibberellins (the abbreviation GA has already been accepted) have been extracted in the pure state from the culture filtrates of G. fujikuroi and higher plants. The basic structure of gibberellins has been well established and found to be derivatives of the aromatic hydrocarbon fluorene with bicyclic unsaturated lactone systems. More than fifty types of gibberellins possessing biological activity are now recognised.
GA1, GA2, GA3 GA4 (gibberellic acid), GA3 and GA7 are highly active. There are some gibberellins with C-20 atoms and some have 19 carbon atoms each. Most gibberellins are monocarboxylic, some are dicarboxylic and very few are tricarboxylic acids.
The different gibberellins vary considerably in their physiological activity. Some like GA8 and GA are highly active, while those like GA8 are almost inactive. Chemical structures of some gibberellins are given in Fig. 746.
Certain GAs have been found in plant tissues to occur in bound or conjugated forms with other compounds—free GAs can be released from bound ones by enzymatic (emulsin) treatment. In pea seedlings, during the first few days of growth, the necessary GA needed for this phase of growth, may partly be released from ‘bound ones’.
Among the bound forms of GAs, the following deserve special mention acetyl GA3, D-glucopyranosyl –GA3, -GA8 -GA26, -GA27, and -GA28.
The most typical and striking plant response to gibberellins is stem elongation, actually internodal elongation, limited exclusively to younger tissues which are still growing (mature tissue is not influenced). It is more active on intact plants than on tissue sections. GA is a growth regulator like IAA which brings about cell elongation but does so by a different mechanism than IAA.
This response may be greatly influenced by external conditions and may result in the change of habit of the plant (forming a vine from a bushy plant or producing a more bushy habit due to outgrowth of laterals). Even, plants like lettuce, which has never been known to show twining habit, have become vines on treating with GA.
There is some evidence that one gibberellin is converted into another within the plant. Thus, there is some evidence to suggest that GA3 is converted into GA1 in pea- seedlings but the extent of this conversion is not nearly enough to account for the spectacular effect of GA5 on growth in this material. Several GAs are probably connected to GA8 in a bound form.
Gibberellin applied in one part of plant or even to the soil can stimulate growth in all the growing parts of the plant.
The number of internodes is not, however, increased nor is the growth exceptionally abnormal or uncontrolled. Major part of GA-effect is attributable to cell elongation and not to any significant increase in cell multiplication.
The most striking effect of gibberellin application is on the growth of the genetical dwarfs (true also for non-genetic dwarfs) which is not duplicated by auxin, IAA. This apparently specific effect of GA in contrast to all other growth-promoting substances on dwarf varieties of pea, Vicia, Phaseolus, etc., (also dwarf mutants of maize) can bring about an increase in growth rates of such dwarf varieties to that of a tall variety.
It is possible to obtain differences in height of 500% in a few days after one application of a few micrograms of gibberellin on a leaflet of dwarf variety of pea. The name gibberellin for this unique growth substance seems singularly appropriate as gibberella in Latin means hunchback or dwarf.
Gibberellins have been isolated from all parts of seedlings of tall and dwarf varieties of pea and also from seeds of pea, French bean, wheat, etc., (there is little doubt now that gibberellins occur naturally in all higher plants).
There is no evidence as yet, however, that gibberellin is found in greater concentration in tall varieties than in dwarf, which might have explained the growth of the dwarf varieties on GA application. Even then it is possible, that in dwarfs, GA is present in a form unable to exert any influence on cell elongation or conceivably there may be inhibitors present which prevent their action.
Another exciting property of gibberellins is their ability to induce flowering in some plants (IAA generally inhibits flowering). In Hyoscyamus, flower formation could be stimulated by GA in an environmental condition under which flowers otherwise would never have formed.
Earlier flowering after gibberellin treatment has been observed in plants in which flowering is normally photo-induced as well as in some species of day- neutral plants.
Usually long-day plants flower in response to gibberellin application; the very few short-day plants which exhibit this response include Impatiens balsamina and a few grasses. After treatment, chrysanthemums were larger and bloomed earlier. Gibberellin nearly doubles the diameter of some Geranium inflorescence. GA usually promotes maleness of flowers.
Gibberellins have been found to substitute for the cold treatment required by biennial long-day plants. Hyoscyamus treated with gibberellin, flowers in the first season without any previous cold treatment. Cold requirement for germination of peach seeds can be annulled by gibberellin treatment.
It has been suggested that GA does not directly affect the internal reactions which take place during a cold vernalisation and that it is certainly not identical with vernalin, the hypothetical product of vernalisation (attempts to vernalise cereal grains with gibberellin have been disappointing).
The cold treatment is replaced by GA, most probably through the removal of the blockage which stands in the way of the internal processes which lead to flowering in cold requiring plants, perhaps by switching on a mechanism entirely different from that utilised normally by low temperature.
It is well known that osmotic inhibition of germination of certain types of seeds can be prevented and actually reversed by giving small quantities of red light of specific spectral quality—660 nm. GA has been found to substitute for red light in protecting lettuce and tobacco seeds against this type of inhibition (see later).
One of the most remarkable effects of gibberellins is that it promotes the induction of many hydrolases, particularly a-amylase and proteinases. In seeds of cereals like barley, wheat or rice these enzymes are produced in the aleurone layers and hydrolyse the starch and protein present in the endosperm to soluble monomers (glucose, amino acids etc.), which are utilized in growth.
Gibberellins promote the growth of leaf bases or the basal region of the leaves. While gibberellins have no effect on the growth of the coleoptile of cereals it promotes the growth of the first leaf which it encloses; the leaf sheath is more sensitive than lamina. These properties are utilized in the bioassay of gibberellins.
Gibberellins do not show the polar transport (they move freely both in the phloem sieve tubes and also in the xylem) within the plant which is characteristic of auxin. In contrast to auxins, GA does not inhibit root growth nor does it cause the typical IAA- epinasty of leaf petiole. Gross deformities and callus formation in plant tissues brought about by IAA are not duplicated by GA.
Gibberellins, however, strongly promote the growth of tissues during certain growth phases that auxins do not. Evidences seem to indicate that the responses of auxins and gibberellins are conditioned by different genes.
In classical auxin bioassay, the Avena coleoptile curvature as also in pea curvature tests, gibberellins have so far consistently given only negative results. The most remarkable of all the differences between GA and IAA appear to be the evident indifference of GA-effect to light direction.
Gibberellins are much more potent than auxins in inducing parthenocarpy (fruit formation without flower fertilisation) and enhancement of fruit yield in grapes and tomatoes. In tomato, seedless parthenocarpic fruits were obtained when the styles of emasculated flowers were treated with, GA mixed with lanolin.
The fruits produced, however, were greatly reduced in size and weight, compared to the normally set, seeded fruits. It is not always necessary that the flower itself be treated; male sterile tomatoes may set fruit when GA is applied to several parts of the plant or even to the soil.
No clear-cut linkage of gibberellin treatment with any metabolic pathway in plants has yet been established but analyses so far have always shown the greatest change among carbohydrate constituents. Sometimes spectacular increase in total sugar concentration has been observed in GA-induced parthenocarpic tomato fruits.
Another apparent effect is the reduction in chlorophyll content accompanying chlorosis caused by application of higher dosages of gibberellin. Promotion of respiration of growing parts of treated plants and seeds has also been reported. Though there are variations in the level of certain enzyme activities, gibberellins themselves are not known to activate isolated enzymes.
There is no convincing evidence, perhaps except one, as yet of any GA-effect on any organism except higher plants including mosses and liverworts. GA itself is apparently destroyed by soil micro-organisms but some reports indicate that the microbial population is modified on GA-application to soil so that growth of the free-living nitrogen-fixing bacteria, Azotobacter, may be favoured in certain types of soil.
Attempts are being made to ascertain the economic importance of gibberellins in agricultural practices. The preliminary results are not very encouraging. Gibberellins have been tried in increasing leaf-expansion in the leafy crops of tobacco and tea.
The most promising avenue for further research still may lie in increasing the yield of hay or jute which may be economically profitable. Spectacular increase in the rate of growth of some trees, ornamental or otherwise, may give interesting results.
D. Ethylene:
The effects of smoke and illuminating gases on plant growth were recognized since the 19th century. The hormonal role of ethylene was first suggested by Crocker, Hitchcock and Zimmermann about fifty years ago.
The most remarkable physiological effect of ethylene is its effect on fruit ripening. Ripening fruits emit ethylene which helps in the ripening of other fruits. Many fruits including bananas, oranges, lemons and apples are ripened this way; however, there are a few exceptions, e.g., peaches and apricots.
Removal of ethylene by a current of air prevents ripening and helps storage; removal of O2, introduction of N2 or CO2 which interferes with its production, also helps in the retention of greenness of fruits. Other physiological effects include inhibition of stem elongation, increased radial expansion— particularly at the basal regions.
Abolition of positive geotropism and promotion of horizontal orientation (presumably due to an interference with polar transport of auxins), stimulation of abscission of leaves and petals, discolouration of flowers, promotion of leaf epinasty (bending of petioles) and hastening of senescence.
Ethylene may be produced from a variety of substances but methionine seems to be the most efficient precursor. Auxin stimulates ethylene production and a number of auxin effects including the early flowering of pine-apple are believed to be mediated through ethylene, ethrel or ethephon, which when sprayed on plants, generate ethylene are used in agriculture and horticulture.
Helminthosporol:
Helminthosporol has been isolated and extracted from cultures of the pathogenic fungus, Helminthosporium sativum by Tamura and others (1965).
Essay # 6. Other Growth-Regulating Substances from Plants:
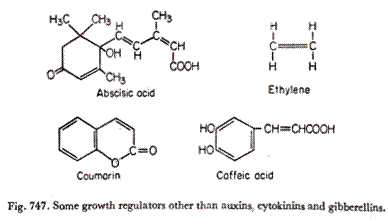
A. Abscisic Acid (ABA):
The first pure abscission inducing substance, isolated and extracted from petroleum ether extract of cotton fruits (1961), has now got the fully approved name, abscisic acid (Fig. 747). It was found to accelerate abscission when applied in very small quantities to the petiole stumps of cotton seedlings.
A second crystalline acidic substance was abscisic acid II, isolated also from young cotton fruits (1963). It also accelerated abscission when applied to petiole bases of cotton seedlings, more effectively than GA3, but had no gibberellin-effect on dwarf maize and was also a strong inhibitor of IAA-induced straight-growth in Avena curvature test.
ABA also promotes senescence and bud and seed dormancy and inhibits seed germination, growth of excised embryos mesocotyls, rice seedlings, expansion of leaf discs and Lemna fronds, regulates stomatal movement, causes turgour loss, and accelerates abscission of excised abscission zones.
It also interacts with other growth hormones. Thus, auxin effects on coleoptile growth and GA3-induction of a-amylase in aleurone layers of barley grains is antagonized by ABA, and the inhibition is reversed by higher concentrations of the growth substance.
ABA-inhibition of the growth of Lemna fronds is also countered by a synthetic cytokinin, benzyladenine. The reversion, however, is not observed in all systems. It enhances colour formation in oranges. ABA effects are rapid and may disappear. ABA is present in all plants, usually in microgram quantities.
It is produced probably by the isoprenoid pathway via mevalonic acid or by catabolic degradation of xanthophylls like violaxanthin. It may be assayed by leaf abscission and induction of bud dormancy tests, Lemna frond growth inhibition and a-amylase induction inhibition tests, but none is specific.
Gas liquid chromatographic techniques, which can detect very small quantities, have been used with advantage by many workers.
B. Traumatig Acid:
Haberlandt’s observation, that in the injured or cut surfaces, some substances were responsible for inducing new cell division to heal up the wound, in 1921 led to the discovery of the so-called, nekrohormones or wound, hormones from the cells bordering the injury.
The following formula was assigned to it:
HOOC.CH = GH. (CH2)8 .COOH
Traumatic acid supplied exogenously is known to induce extensive wound periderm formation in washed potato tuber.
Perhaps Went’s (1938) postulated existence of calines should be mentioned here. Most of the evidence for their existence in plants is indirect (they have never as yet been identified in plants) and the adenine derivative of cytokinin, zeatin has effects on plants analogous to those of the postulated calines.
The various postulated calines are:
(i) Rhizocaline, synthesised in the leaves or stored in the seeds and necessary for root formation; they are thought to be translocated primarily downwards;
(ii) Caulocaline; synthesised in roots or it may be stored in the seeds but necessary for elongation of stems—translocated primarily upwards; and
(iii) Phyllocaline, made or stored in the cotyledons and supposed to be necessary for leaf growth.
Essay # 7. Synthetic Growth Regulators in Plants:
A. Growth Retardants:
Many synthetic organic compounds (Fig. 748) are now known which antagonise or even nullify the longitudinal growth promotion induced by GA in plants.
They appear to reduce stem elongation by altering plant metabolism and hence blocking GA biosynthesis (antimetabolites), rather than by acting as competitive antagonists to GA as well as to IAA in their growth processes. These compounds inhibit one or more specific steps of GA biosynthesis.
Plants become short and bushy but greener. Flowering in some plants, e.g., azaleas and rhododendrons is accelerated. With cycocel biennial mango plants have been found to flower in the off-season. Cycocel also promotes the formation of female flowers in Papaya. They are usually called growth retardants.
Among the specific GA-antagonists, are Phosphon D (tributyl-2, 4-dichlorobenzyl phosphonium chloride), AMO 1618, CCC, etc. All of them are complex chlorides.
B. Morphactins:
a chemical group of regulator substances, morphactins (morphologically active substances) has been synthesised by 1960 in E. Merck’s Laboratories. Since 1964-65, this group, consisting of fluorene-9-carboxylic acids and their derivatives, has been the subject of considerable interest as a representative or new type of growth regulator.
Their action is systemic, but slow, and can be transported both basically and acropetally (as in GA). These non-toxic substances inhibit and modify development and new growth.
The morphogenic action of morphactins begins only with higher plant groups, starting with highly organised brown algae, the mosses and ferns. Unicellular plants and simple cell filaments are not affected by morphactins.
Morphactins show some similarities of their action with ABA.
However, some of the effects may be considered characteristic for morphactins, which are not duplicated by any other synthetic or natural growth regulators.
They are really unique in bringing about these responses, as for example, the complete and striking abolition of polarity phenomena in plants by morphactins; the reduction of apical dominance of the main shoot; promotion of shoot branching; reinforcement of the apical dominance of the tap roots; the abolition of gravity and photo-induced directional growth—geotropism and phototropism; some disturbance, difficult to specify, of the coordination and correlation phenomena and the action on cell division; the inhibition of mitoses in apical meristems; stimulation of cell division in cambium and pericycle of intact plants and cuttings, etc. Morphactin effects may disappear after withdrawal.
C. Inhibitors:
Various phenolics, coumarins (Fig. 748) and cucurbitacins (abundant in the family Cucurbitaceae) are some of the naturally occurring inhibitors which affect several physiological processes including germination, growth, flowering and senescence in addition to metabolic processes.
Essay # 7. Mechanism of Action of Growth Regulators in Plants:
Plant growth regulators have varied physiological effects and each of them produces multiple effects, depending on the concentration used and the organ under study. Some, like auxins, have fast and delayed actions.
The morphogenetic changes brought about take quite some time and involve the formation of new enzymes and structural proteins. This implies an interaction with the genomal DNA. Receptor molecules for some hormones have been claimed to have been isolated, as in the case of animal hormones.
Hormones are believed to interact with some molecules, presumably proteins, which then interact with the chromatin thereby influencing transcription. Direct inter- action between auxin, GA and cytokinin on the one hand and DNA, RNA and protein on the other hand have also been demonstrated, although their biological significance has not been adequately explained.
In the case of gibberellins, synthesis of the a-amylase enzyme protein in response to GA3 application has been demonstrated.
The fast action of auxin is evident within a few minutes of application. Since growth is enhanced by dilute acid solution, it has been suggested that auxin in some way influences membrane potential and activates a “proton pump“— the active process concerned with transport of H+-ions across membranes.
There H+-ions probably weaken the H-bonds connecting xyloglucan molecules with cellulose fibrils in the primary cell wall. In fact some xyloglucan is liberated in pea stem sections as growth is promoted by IAA.
As the wall is loosened, more water is taken up by the cell due to enhanced osmotic pressure or decreased water potential arising as a consequence of hydrolysis of some cell substance or enhanced ionisation of some electrolyte. With increase in cell volume the membrane presses against the loosened wall.
New wall material including polysaccharides and proteins are gradually laid down so that the wall regains its rigidity after some time. The proton pump hypothesis, however, has been questioned recently and a more satisfactory explanation is necessary.
Cytokinins which stimulate cell division and delay senescence are known to promote DNA, RNA and protein synthesis is several tissues, although the mechanism of this stimulation is not understood. Some nucleotides of t-RNA have cytokinin activity; it is not known whether they help in the assembly of amino acids on the m-RNA- ribosome complex.
Cytokinins probably influence the activity of certain enzymes— particularly hydrolases and those which synthesise certain aminoacids.
ABA has also been suspected to affect nucleic acid and protein metabolism in some way but no conclusive evidence is available. Its rapid effect or stomatal movement is suggestive of other sites of action.
The effects of various growth substances overlap and they may also interact indirectly. In some cases the effect of one has been found to be duplicated by another. While this may be mediated through the transcription-translation machinery, other mechanisms are also possible, though evidence the present moment is not available.