In this essay we will discuss about Photosynthesis in Plants. After reading this essay you will learn about: 1. Meaning of Photosynthesis 2. Significance of Photosynthesis to Mankind 3. History 4. Photosynthetic Apparatus 5. Pigments 6. Quantum Requirement and Quantum Yield 7. Mechanism 8. Evidences for Existence of Light and Dark Reactions 9. Source of Oxygen 10. Factors Affecting.
Contents:
- Essay on the Meaning of Photosynthesis
- Essay on the Significance of Photosynthesis to Mankind
- Essay on the History of Photosynthesis
- Essay on the Photosynthetic Apparatus
- Essay on the Photosynthesis Pigments
- Essay on the Quantum Requirement and Quantum Yield
- Essay on the Mechanism of Photosynthesis
- Essay on the Evidences for Existence of Light and Dark Reactions in Photosynthesis
- Essay on the Source of Oxygen Released in Photosynthesis
- Essay on the Factors Affecting Photosynthesis
Contents
- Essay # 1. Meaning of Photosynthesis:
- Essay # 2. Significance of Photosynthesis to Mankind:
- Essay # 3. History of Photosynthesis:
- Essay # 4. Photosynthetic Apparatus:
- Essay # 5. Photosynthesis Pigments:
- Essay # 6. Quantum Requirement and Quantum Yield:
- Essay # 7. Mechanism of Photosynthesis:
- Essay # 8. Evidences for Existence of Light and Dark Reactions in Photosynthesis:
- Essay # 9. Source of Oxygen Released in Photosynthesis:
- Essay # 10. Factors Affecting the Photosynthesis:
Essay # 1. Meaning of Photosynthesis:
Although literary meaning of photosynthesis is ‘synthesis with the help of light’ but this term is usually applied to a very important vital process by which the green plants synthesize organic matter in presence of light. Photosynthesis is sometimes called as carbon assimilation and is represented by the following traditional equation.
In recent years, this equation has more appropriately been modified as follows:
During the process of photosynthesis, the light energy is converted into chemical energy and is stored in the organic matters which is usually the carbohydrate and along with O2 form the end products of photosynthesis. One molecule of glucose (C6H12O6) for instance, contains about 686 k. cal. (2868 kJ) of energy. CO2 and H2O constitute the raw materials for this process. It is an anabolic process.
About 90% of the total photosynthesis in the world is carried out by algae growing mainly in oceans and also in fresh water.
Essay # 2. Significance of Photosynthesis to Mankind:
i. It maintains equilibrium of O2 in the atmosphere.
ii. It provides food either directly as vegetables, or indirectly as meat or milk of animals which in turn are fed on plants.
iii. Besides providing energy in the form of food, photosynthesis has also provided vast reserves of energy to man as fuel such as coal, oil, peat and also wood and dung.
Essay # 3. History of Photosynthesis:
The history of photosynthesis dates back to about 1648 when a Dutchman Van Helmont planted a 5 pounds willow shoot in 200 pounds of dried soil. After 5 years of watering with rain water the willow tree weighed 169 pounds. When the soil was dried and again re- weighed, it was found to have lost only 2 ounces. He suggested that the increase in the plant substances of the willow tree must have come from water alone. Prior to this and from the time of Aristotle the idea was prevalent that the plants feed on humus.
Stephan Hales (1727) pointed out that the plants obtained a part of their nutrition from the air and also suggested that sunlight may play a role in it.
Priestley (1772) showed that the plants might restore the air which has been “injured” (i.e., laden with CO2) by the burning of candles.
Ingenhousz (1779) noticed that only the green parts of the plants were able to purify the air and that too in the presence of sunlight.
Jean Senebier (1783) noted that the air-purifying activity of plants depends on the presence of fixed air (i.e., CO2) and suggested that the air (O2) liberated by plants which are exposed to sunlight is the product of the transformation of fixed air (CO2) by sunlight.
Nicolas Theodore de Saussure (1804) showed that the total weight of the organic matter produced and oxygen evolved by the green plants in presence of sunlight was greater than the weight of fixed air (CO2) consumed by them during this process. He concluded that besides fixed air (CO2) water must constitute the raw material for this process.
In 1845 Meyer recognised the role of light as a source of energy and thus it became possible to formulate the overall process of photosynthesis as conversion of water, CO2 and light energy into O2 and organic matter containing chemical energy by the green plants and which could be represented by the following equation.
In 1862 Julius Sachs showed that the process of photosynthesis takes place in chloroplasts and results in the synthesis of starch (organic matter). Although, the general outline of photosynthesis was complete by the middle of 19th century, but it took almost another century before the modern methods of microscopic and radiochemical analysis yielded structural and chemical details of photosynthesis.
Essay # 4. Photosynthetic Apparatus:
The chloroplasts in green plants constitute the photosynthetic apparatus. Typically, the chloroplasts of higher plants are discoid or ellipsiodal in shape, 4-6µ in length and 1-2µ thick. The chloroplast is bounded by two membranes each app. 50 Å thick and consisting of lipid bilayer and proteins. The thickness of the two membranes including the space enclosed by them is app. 300 Å.
Internally the chloroplast is filled with a hydrophilic matrix called as stroma in which are embedded grana. Each granum has a diameter of 0.25-0.8µ and consists of 5-25 disk shaped grana lamellae placed one above the other like the stack of coins (Fig. 11.1 A). In cross section these lamellae are paired to form sac like structures and have been called as thylakoids. Each grana lamella or thylakoid encloses a space, the loculus or lumen.
The ends of disk-shaped thylakoids are called as margins (which are fused to form sac or lumen like structure) while the contiguous membranes between two thylakoids form the partition. Some of the grana-lamellae or thylakoids of a granum are connected with thylakoids of other grana by somewhat thinner stroma-lamellae or fret membranes.
These also enclose spaces which are called as fret-channels (Fig. 11.1 B). Thylakoid membranes and stroma lamellae both are composed of lipid bilayer and proteins.
Chlorophylls and other photosynthetic pigments are found in the form of protein pigment complexes mainly in thylakoid membranes of grana. The latter are sites of primary photochemical reaction. Some of the protein-pigment complexes are also found in stroma lamellae.
Dark reaction of photosynthesis occurs in stroma. Besides necessary enzymes, some ribosomes and DNA have also been found in chloroplasts which give them (chloroplasts) a partial genetic autonomy.
Essay # 5. Photosynthesis Pigments:
Photosynthetic pigments are of three types:
(1) Chlorophylls,
(2) Carotenoids, and
(3) Phycobillins.
i. Chlorophylls and carotenoids are insoluble in water and can be extracted only with organic solvents.
ii. Phycobillins are soluble in water.
iii. Carotenoids include carotenes and xanthophylls. The latter are also called as carotenols.
iv. Different pigments absorb light of different wavelengths and characteristic absorption peak in vivo and in vitro.
v. They show property of fluoresces.
Distribution of Photosynthetic Pigments in Plant Kingdom:
The distribution of the different types of photosynthetic pigments in plant kingdom is shown in table 11.1.
A new form of chlorophyll has been discovered recently by Chen et al (2010) from stromatolites of Shark Bay in Western Australia which they have called as chlorophyll f. This pigment is believed to absorb light upto 706 nm in vitro, with a fluorescence of 722 nm. (stromatolites are structures formed from layers of cyanobacteria (blue-green algae), and other microorganisms, calcium carbonate and sediments).
Structure of Photosynthetic Pigments:
(1) Chlorophylls:
They are magnesium porphyrin compounds. The porphyrin ring consists of four pyrrol rings joined together by CH bridges. A long chain of C atoms called as phytol chain is attached to porphyrin ring at iv pyrrol ring.
I. Chemical structures of chlorophyll-a and chlorophyll-b are well established.
II. Molecular formulae of chlorophyll-a and chlorophyll-b are C55H72O5N4Mg and C55H70O6N4Mg respectively.
III. Molecular structure of chlorophyll-a and b are given in Fig. 11.2 A. Both of them consist of Mg-Ppyrrolorphyrin ‘head’ which is hydrophilic and a phytol ‘tail’ which is lipophilic. The two chlorophylls differ because in chlorophyll-b there is a -CHO group instead of a -CH3 group at the 3rd C atom in II ring.
IV. The phytol (C20 H39OH) is a 20-C isoprenoid alcohol that is esterified to the IV pyrrole ring of the chlorophyll molecule and anchors it in the photosynthetic membrane.
V. The structural formulae of chlorophyll-c and chlorophyll-d are also now known. Chlorophyll-c differs from chlorophyll-a in lacking the phytol tail. Chlorophyll-d differs from chlorophyll-a in that instead of -CH = CH, group, there is a – O – CHO group at 2nd C-atom in the 1st pyrrole ring.
VI. Pheophytin resembles chlorophyll-a in structure except that it lacks Mg atom, which is replaced by two H atoms (Fig. 11.2 B).
VII. Chlorophyll is formed from protochlorophyll in light. The protochlorophyll lacks two hydrogen atoms one each at 7th and 8th C atoms in IV pyrrole ring.
Protochlorophyll Light/2H→ Chlorophyll
This reaction is catalyzed by the enzyme NADPH: protochlorophyll oxidoreductase.
Systematic studies by R.M. Willstatter in 1910s and later by Hans Fischer (both German Scientists and Nobel Laureates of Chemistry in 1915 and 1930 respectively) established the molecular structure of chlorophyll. The structural formula for chlorophyll determined earlier by Hans Fischer in 1940 was confirmed by Complete Synthesis of the molecule by Woodward in 1960.
(2) Carotenoids (Yellow or orange pigments):
(i) Carotenes:
I. These consist of an open chain conjugated double bond system ending on both sides by ‘ionone’ rings.
II. They are hydrocarbons with general formula C40H56.
III. Different carotenes differ only in the arrangement of their molecules in space i.e., they are stereoisomers. Structural formulae of some carotenes are given in Fig. 11.3A.
IV. In lycopene, which is chief pigment of tomato fruit, the ring at both the ends of its structure are open and not closed.
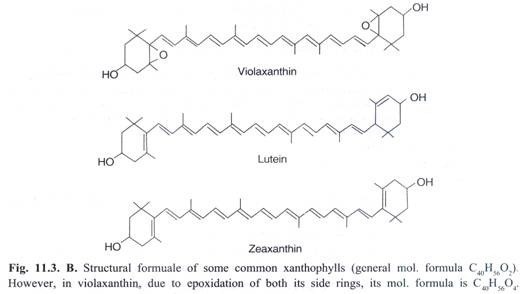
(ii) Xanthophylls (Carotenols):
These are similar to carotenes but differ in having two oxygen atoms in the form of hydroxyl, carbonyl, or carboxyl groups attached to the ionone rings. Accordingly, their general formula is C40H56O2. In some xanthophylls such as violaxanthin, due to epoxidation of rings, the general formula becomes C40H56O4. Structural formuale of some of the xanthophylls are given in Fig. 11.3B.
i. In autumn, just before leaf fall, chlorophylls are degraded unmasking the more stable carotenoid pigments which impart brilliant yellow and orange colour to the foliage.
Apart from their role in absorption of light energy and its transfer to chlorophyll-a, the carotenoids play a very important role in preventing photodynamic damage within the photosynthetic apparatus.
Photodynamic damage is caused by oxygen molecules in their first singlet state which is very reactive and is capable of oxidising whole range of organic compounds such as chlorophylls and thereby making them unfit (damaging) for their normal physiological functions.
Carotenoids can prevent this photodynamic damage (i) by quenching the first excited triplet state of the chlorophyll photosynthesizer (ii) by quenching singlet oxygen directly and (iii) rarely, some of the carotenoid molecules may act as substrate for oxidation by singlet oxygen which may have left in (i) and (ii).
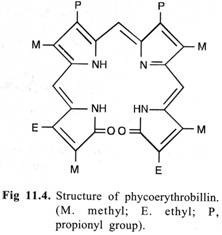
(3) Phycobillins (Red and Blue Pigments):
These consist of an open conjugated system of four pyrrol rings and lack Mg and the phytol chain. The structure of the red pigment phycoerythrobillin is given in Fig. 11.4. Phycobiliproteins are organized into larger macromolecular complexes in the cell and are called as phycobilisomes.
Location of Photosynthetic Pigments in Chloroplasts:
According to the classical unit membrane model of cell membranes, the photosynthetic pigments were thought to be located in grana portions of the chloroplasts in higher plants. A number of molecular models of the chloroplasts showing the arrangement of pigment molecules were given by different workers from time to time and it was usually held that chlorophyll molecules formed a monomolecular layer between the alternative protein and the lipid layers in grana lamellae (thylakoids)). The hydrophillic ‘heads’ of the chlorophyll molecules remain embedded in the protein layer while the lipophilic phytol ‘tails’ in the lipid layer (Fig. 11.5).
The other pigments were thought to be present along with the chlorophyll molecules. Weier and Benson (1966, 1967) had also included chlorophyll molecules in the fret membranes (stroma lamellae) in their model of the chloroplasts.
In recent years the Fluid Mosaic Model of cell membranes has been widely recognised. Accordingly, the perception regarding the location of photosynthetic pigments in lamellar membranes within the chloroplasts has also been changed.
It is now widely accepted that the photosynthetic pigments occur as protein-pigment complexes as parts of photosytems (pigment systems) I and II which are dispersed in the lipid bilayer of thylakoid membranes of grana. They may also be present in stroma lamellae (Fig. 11.6).
Absorption and Utilisation of Light Energy by Photosynthetic Pigments:
i. Chief source of light energy for photosynthesis is sun.
ii. The earth receives only about 40% (or about 5 × 1020 k.cal.) of the total solar energy. The rest is either absorbed by the atmosphere or is scattered into space.
iii. All the incident light energy falling on green parts of the plants is not absorbed and utilised by pigments. Some of the incident light is reflected, some is transmitted through them while only a small portion is absorbed by the pigments.
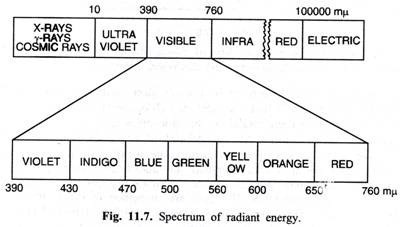
iv. Photosynthetic pigments absorb light energy only in the visible part of the spectrum ranging usually between 400-700 mµ (nm). Such radiations are called as photosynthetically active radiations (PAR). However, certain photosynthetic bacteria use infra-red light of comparatively shorter wavelengths. The spectrum of radiant energy is given in Fig. 11.7.
v. (In modern scientific literature, some plant physiologists equate PAR with visible part of spectrum of radiant energy which is erroneous. This is because such scientists working on photobiology use commercially available instruments that are limited to that portion of spectrum between 400-700 nm only, thus excluding visible light in the 700-760 and 390-400 nm range.)
vi. Only about 1% of the total solar energy received by the earth is absorbed by the pigments and is utilised in photosynthesis.
vii. There is very weak absorption by pigments in green part of the spectrum and hence, the chloroplasts appear green in green plants.
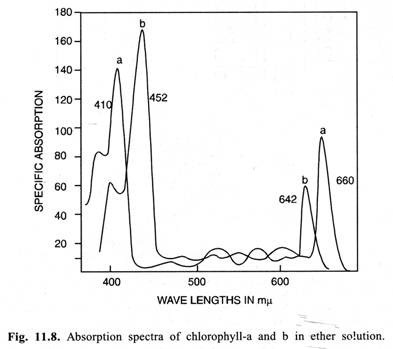
Absorption Spectra of Chlorophylls:
They chiefly absorb in the violet-blue and red parts of the spectrum. The absorption band shown by the chlorophylls in violet-blue region is also called as soret band. Characteristic absorption peaks shown by different chlorophylls both in vivo (i.e., intact cell) and in vitro (i.e., in solvents) are given in Table 11.2.
i. Absorption spectra of different chlorophylls differ in vivo and in vitro.
ii. From Table 11.2 it is quite evident that there are several forms of the chlorophyll-a in vivo showing a number of absorption peaks in the red part of the spectrum.
Absorption spectra of chlorophyll-a and chlorophyll-b (in-vitro) are given in Fig. 11.8.
Absorption Spectra of Carotenoids:
These pigments absorb light energy in blue, blue- green and green parts of the spectrum.
Absorption Spectra of Phycobillins:
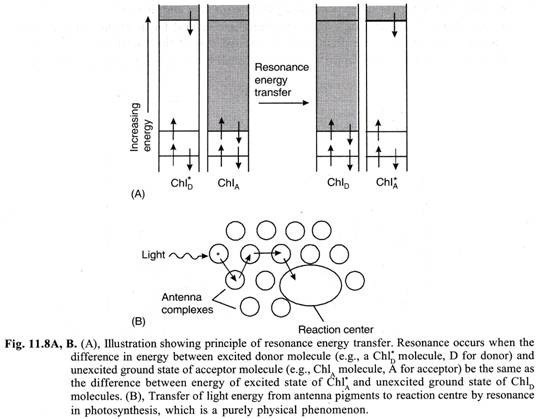
Transfer of Light Energy Absorbed by Accessory Pigments to Chlorophyll-a:
All the pigments except chlorophyll-a are called as accessory pigments or antenna pigments. Almost all (app. 95-99%) the light energy absorbed by accessory pigments is transferred to chlorophyll-a molecule by resonance (also known as Forster transfer) which alone can take part in primary photochemical reaction in photosynthesis (Fig. 11.8).
Chlorophyll-a molecules also absorb light energy directly. However, in photosynthetic bacteria the above function is accomplished by bacteriochlorophyll and bacterioviridin because they do not have other types of pigments.
Only special forms of chlorophyll-a (viz., p680 and p700) can take part in primary photochemical reaction in photosynthesis that constitute the reaction centres in PSII and PSI respectively. All other forms of chlorophyll-a, therefore, also come under the purview of accessory pigments.
Similarly, in photosynthetic bacteria, only special forms of bacteriochlorophylls called P840 and P870 are known to constitute the reaction centres.
Essay # 6. Quantum Requirement and Quantum Yield:
The number of photons (or quanta) required to release one molecule of oxygen in photosynthesis is called as quantum requirement. On the other hand, the number of oxygen molecule released per photon of light in photosynthesis is called as quantum yield. The quantum yield is always in fraction of one.
Warburg found minimum quantum requirement for photosynthesis to be 4. It is because the reduction of one molecule of CO2 by two molecules of H2O requires the transfer of 4 H atoms. The transfer of each H atoms from H2O to CO2 requiring one photon or quantum of light.
(CH2O) in the above equation represent 1/6 of the carbohydrate molecule such as glucose. One molecule of glucose molecule contains about 686 k. cal. of energy, therefore, 1/6 glucose molecule will contain 686/6 = app. 112 k. cal. of energy. We also know that the rate of photosynthesis is maximum in red light and each photon of red light contains about 40 k. cal. of energy.
This would suggest that the efficiency with which the plants can convert light energy into chemical energy is 112/40 × 4 = 70% which indeed is very high. Warburg has even reported that Chlorella can carry out photosynthesis with a quantum requirement of only 2.8 i.e., with cent per cent efficiency of converting light energy into chemical energy.
Warburg’s findings have been severely criticised by Robert Emerson and other workers. According to them photosynthesis is a very complicated process and is not so efficient as to convert all the light energy into chemical energy.
There is a considerable loss of light energy absorbed during photosynthesis, therefore, the minimum quantum requirement for photosynthesis as suggested by them is 8—10 which is widely accepted at present. Considering that the quantum requirement for photosynthesis is 8-10, the quantum yield would accordingly be 1/8 = 0.125 to 1/10 = 0.10.
Essay # 7. Mechanism of Photosynthesis:
The process of photosynthesis is a complicated oxidation reduction process resulting ultimately in the oxidation of water and reduction of CO2.
The mechanism of photosynthesis consists of two parts:
(1) Primary photochemical reaction or Light reaction or Hill’s reaction and
(2) Dark reaction or Blackman’s reaction or Path of carbon in photosynthesis.
In Primary photochemical reaction assimilatory power (i.e., NADPH + H+ + ATP) is generated and O2 is released. The assimilatory power is utilised in dark reaction in reducing CO2 to carbohydrates. Water is ultimate electron donor in photosynthesis.
(1) Primary Photochemical Reaction or Light Reaction or Hill’s Reaction:
Primary photochemical reaction which is faster than the dark reaction, takes place only in presence of light in the grana portions of the chloroplasts.
It can be studied under the following heads:
(i) Absorption of Light Energy by Chloroplast Pigments:
Different chloroplast pigments absorb light in different regions of the visible part of the spectrum.
(ii) Transfer of Light Energy from Accessory Pigments to Chlorophyll-a:
All the photosynthetic pigments except chlorophyll-a are called as accessory or antenna pigments. The light energy absorbed by them is transferred by resonance to chlorophyll-a which alone can take part in primary photochemical reaction. Chlorophyll-a molecule can also absorb the light energy directly. In pigment system II the photoreaction centre is P 680 (or in some plants P 690) while in pigment system I it is P 700.
(iii) Activation of Chlorophyll-a Molecule by Photons of Light:
When P 680 or P 700 forms of chlorophyll-a molecule in two pigment systems receives a photon (quantum) of light it becomes an excited molecule having more energy than the ground state energy. After passing through the unstable second singlet state and first singlet state the chlorophyll molecule comes to the metastable triplet state. It is this latter excited from of chlorophyll-a which in-fact takes part further in primary photochemical reaction.
It expels its energy along with an electron and a positive charge comes on the chlorophyll-a molecule which now becomes oxidised:
Chlorophyll-a Light→ Excited Triplet State of Chlorophyll-a
Excited Triplet State of Chlorophyll-a → (Chlorophyll-a)+ + e–
(The Mg-porphyrin ‘head’ region of the chlorophyll molecule absorbs light that is also site of electron rearrangements which occur when the chlorophyll is excited and of the unpaired electrons when it is either oxidized or reduced).
(iv) Photolysis of Water and O2 Evolution (Oxidation of Water):
These are associated with oxygen evolving complex (OEC) or water splitting complex in pigment system II and are catalysed by presence of Mn++ and Cl– ions. When pigment system II is active i.e., it receives light, the water molecules split into OH– and H+ ions (photolysis of water). Its mechanism is least understood.
The OH– ions unite to form some water molecules again and release O2 and electrons. It is believed that photolysis of water involves a strong oxidant which is yet unknown and is designated as Z (or Yz).
There are now strong evidences to suggest that liberation of one O2 molecule from 2H2O molecules is a four steps process. The unknown strong oxidant ‘Z’ is supposed to form a redox-system between H2O and P 680+ which must accumulate four positive charges before a molecule of O2 is evolved. Each step requires one extremely short flash of light or a photon (hv) in which one positive charge is added on ‘Z’.
This can be explained further by a schematic model for the photo-oxidation of water given by Bessel Kok et al (1970) which is widely accepted and is called as S state mechanism or sometimes as water oxidizing clock. It consists of a series of 5 states called as S0, S1, S2, S3 and S4 which represent successively more oxidised forms of the water oxidizing system or oxygen evolving complex (OEC) S0 is uncharged state.
Each short flash of light (photon or hv) converts S0 to S1, S1 to S2, S2 to S3 and S3 to S4. After the S4 state has acquired four positive charges, it gets four electrons back in one step oxidation of two molecules of H2O and returns back to S0with four fewer charges than S4 (fig. 11.14).
However, the chemical nature of S state in this ‘clock’ is yet unknown. Once it was believed that P680 becomes oxidised by loss of one electron after a brief flash of light to P680+ but P680 cannot be S because it can lose only one electron and can accumulate only one positive charge.
Later studies have shown that various S states probably represent oxidation states of manganese including Mn2+, Mn3+ and Mn4+. This hypothesis has received strong support from a variety of experiments, especially X-ray absorption and ESR studies which detect the manganese directly (Yano at al, 2006).
It is now known that the immediate electron donor to PSII is a tyrosine (an amino acid) residue which is often designated as Z or Yz in subunit D1 of PSII reaction centre. (Y is code letter for tyrosine; hence Z is now called as Yz). It is believed that tyrosine radical regains its electron by oxidizing a cluster of 4 Mn ions in OEC.
With each single electron transfer, the Mn cluster becomes more oxidized. Four single electron transfers (each corresponding with one photon (hv) of light) produce four positive charges on Mn cluster. In this state, Mn complex can take four electrons (4e-) from a pair of water molecules. The exact mechanism of photo-oxidation of H2O2 however, remains elusive.
(The OEC is a 33kD complex situated on lumenal side of thylakoid. The 4H+ released by photolysis of 2H2O molecules are released into lumen of thylakoid where they add to the proton gradient necessary for photophosphorylation. Apart from Mn2+ and Cr ions, Ca2+ ions are also believed to be essential for photolysis of water.)
(v) Electron Transport and the Production of Assimilatory Power (i.e., NADPH + H+ + ATP):
It has already been said that when chlorophyll-a molecule receives a photon of light it becomes excited and expels the extra energy along with an electron in both the pigment systems. This electron after travelling through a number of electron carriers is either cycled back or is consumed in reducing NADP+ (Nicotinamide Adenine Dinucleotide Phosphate) to NADPH + H+.
The extra light energy carried by the electron is utilised in the formation of ATP molecules at certain places during its transport. This process of the formation of ATP from ADP and inorganic phosphate (Pi) in photosynthesis is called as photosynthetic phosphorylation or photophosphorylation. Arnon has contributed a lot in our understanding of the electron transport and photophosphorylation in chloroplasts.
These are of two types:
(a) Non-cyclic Electron Transport and Non-cyclic Photophosphorylation (Z-Scheme):
This process of electron transport involves both PSI and PSII which act in tandem or series and is initiated by the absorption of a photon (quantum) of light by P700 form of chlorophyll- a molecule in pigment system I which gets excited. An electron is ejected from it so that an electron deficiency or a ‘hole’ is left in the P700 molecule (or in other words a positive charge comes on chlorophyll-a-molecule).
This ejected electron is trapped by FRS (Ferredoxin reducing substance) which is an unknown oxidation-reduction system with a redox potential (E0‘) of -0.6 volts and may be a pteridene. The electron is now transferred to a non-heme iron protein called ferredoxin (Fd) with E’0 of-0.432 V. From ferredoxin the electron is transferred to NADP (E0‘ = -0.32 V) via intermediate protein electron carrier ferredoxin-NADP reductase (FNR) so that NADP is reduced to NADPH + H+.
Most recent researches have shown that FRS is in-fact a series of electron carriers which in their reduced form are very unstable and difficult to be identified and are designated as A 0 A 1 Fe-S1 ,Fe-SA & Fe-SB. A0 is probably a chlorophyll molecule that receives electron from P700.
A1 is believed to be phylloquinone (vit. K1). Fe-Sx, Fe-SA and Fe-SB are iron-sulphur centres situated on proteins in core complex I (CCI) and act as additional electron carriers. From Fe-S centres, the electron is transferred to ferredoxin (Fd) which is a small, water soluble iron-sulphur protein situated on stroma side of thylakoid membrane (Fig. 11.16).
Now, when a photon (quantum) of light is absorbed by P680 form of chlorophyll-a molecule in pigment system II, it gets excited and an electron is ejected from it so that an electron deficiency or a ‘hole’ is left behind in the P680 molecule. The ejected electron is trapped by a compound of unknown identity usually designated Y (Compound Y is sometimes called as Q because it also causes quenching of the characteristic fluorescence of chlorophyll-a in pigment system II).
This unknown compound forms oxidation-reduction system with a redox-potential (E0‘) value more negative than 0.0 V. From Q the electron passes downhill along a series of compounds or intermediate electron carriers and is ultimately received by pigment system I where it ‘fills the hole.’ Redox potential of P700 in pigment system is + 0.43 V.
The series of compounds consists of (i) cytochrome b-559 (E0‘ = + 0. 055 V), (ii) plastoquinone (PQ) whose chemical structure shows similarity with vitamins of K Series. It has a redox potential (E0‘) of + 0.113 V, (iii) cytochrome ƒ (E0‘ = + 0.36 V) and (iv) plastocyanin (PC) which is copper containing protein (E0‘ = + 0.39 V).
At one place during the electron transport i.e., between plastoquinone and cytochrome ƒ there is enough change in free energy which allows phosphorylation of one molecule of ADP to form one ATP molecule (photophosphorylation).
Most recent researches have shown that from p680, the electron is transferred to unknown compound ‘Q’ via pheophytin. The latter is special form of chlorophyll-a which lacks magnesium atom (Fig. 11.2B). The unknown compound Q exists in two forms QA & QB.
It is now known that QA and QB are infact specialized plastoquinones (PQ) which receive electron from pheophytin and transfer it to Cyt. b6f complex. QA is attached strongly to D2 protein, while QB is attached loosely to D1 protein in core complex II (CC II). After the QB has received two electrons from QA (one by one in two turns), it also takes two protons (2H+) from stroma and is fully reduced to uncharged plastoquinol or plastohydroquinone (PQH2 or PQBH2).
The PQH2 is now released from the reaction centre and is replaced by another molecule of PQ which now occupies the QB site (11.16). From PQH2, electrons are transferred to cytochrome b6f complex and its two protons (2H+) are expelled into the lumen of thylakoid. Finally, the electrons from Cyt b6f complex reach to PSI via plastocyanin (PC).
(It is important to note that QA is one electron acceptor, while QB is two electrons acceptor).
i. Cytochrome ƒ is a typical c type of cytochrome, ‘ƒ’ is abbreviated from ‘frons’ which in Latin means leaf).
The ‘hole’ in pigment system I has been filled by the electron coming from pigment system II. But the ‘hole’ or an electron deficiency is still there in pigment system II. This is fulfilled by the electron coming from photolysis of water. Water here acts as electron donor. It has redox-potential (E’0) of +0.82 V. This transfer of electron from water probably involves a strong oxidant which is yet unknown and is designated as Z or Yz.
In the above scheme of electron transport the electron ejected from pigment system II did not return to its place of origin, instead it was taken by pigment system I. Similarly, the electron ejected from pigment system I did not cycle back and was consumed in reducing NADP+. Therefore, this electron transport has been called as non-cycle electron transport and the accompanying photophosphorylation as non-cyclic photophosphorylation.
ii. Arrangement of PSI and PSII and various components of non-cyclic electron transport chain when depicted on paper according to their redox-potential values, takes a zig-zag shape like the letter ‘Z’ (Fig. 11.15) hence, non-cyclic electron transport is also called by the name Z-scheme.
The idea of Z-Scheme of photosynthetic electron transport was first given by Hill & Bendall (1960). Fig. 11.15A shows current view of Z-Scheme.
iii. Non-cyclic photophosphorylation and O2 evolution are inhibited by 3-(4′-chlorophenyl)- 1, 1-dimethylurea (CMU) and 3-(3’4′-dichlorophenyl)-1, 1-dimethylurea (DCMU).
(In the electron transport system described above we have considered the transport of only one electron because P700 and P680 molecules in pigment systems I & II respectively can give or take only one electron at a time. But the reduction of one molecule of NADP+ (NADP+ → NADPH + H+) requires 2 electrons and 2 protons. Therefore, two molecules of P700 are excited by 2 photons (quanta) of light to release 2 electrons which reduce NADP+.
Similarly two photons will release 2 electrons from two molecules of P680 which in turn will be taken by two molecules of P700 in pigment system I. On the other hand, photolysis of one molecule of H2O results in the production of 2 electrons, 2 protons and ½O2. These 2 electrons thus produced will fill the holes of two molecules of P680 in pigment system II while 2 protons (H+) will be utilised in the reduction of NADP+. Thus 4 photons and one water molecule are required to reduce one molecule of NADP+.
Like P700 and P680 molecules, the cytochromes and plastocyanin involved in electron transport also have only one electron transition. Plastoquinone on the other hand has 2 electron transition i.e., it can either give or take two electrons at one time along with two protons).
Charge separation:
In PSII, from excited P680* molecule, the electron with extra energy is transferred to pheophytin within 3 picoseconds (pico = 1012) resulting in the formation of P680+ and Pheo–. This is called as charge separation. This charge separation effectively stores the light energy of the photon as redox energy and represents the actual conversion of light energy into chemical energy.
The charge separation is stabilized (and the backward reaction is prevented) by rapid transfer of electron within 250-500 microseconds from Pheo– to neighbouring QA and the instant reduction of oxidized P680+ by a redox-active tyrosine (an amino acid) residue Yz of the D1 protein in PSII.
Mechanism of photophosphorylation:
As mentioned earlier, plastoquinone (PQ or PQB) is in-fact a hydrogen carrier. When it is reduced by two electrons (one by one in two turns) by PQA, two protons (2H+) one also taken by it from aqueous stroma of the chloroplast, so that it is converted into fully reduced uncharged form (PQH2 or PQBH2). In turn, when PQBH2 transfers electrons to plastocyanin (PC) through Cyt b6 f complex, 2H+ ions are released into the lumen of thylakoid (Fig. 11.16).
Thus as the two electrons flow down the intermediate electron transport chain through PQ ⇋ PQH2 system or pool, two H+ ions are pumped across the thylakoid membrane. The resulting H+ gradient (high conc. of H+ inside thylakoid and low conc. of H+ outside) is believed to be the main driving force for the conversion of ADP + Pi into ATP by anisotropic ATPase (ATP-Synthase) which is located in the thylakoid membrane.
The protons diffuse down their proton electro-chemical gradient across the thylakoid membrane through proton channels in CF0 complex and result in synthesis of ATP on catalytic sites situated on F1 complex. The protons coming from splitting of water molecules in photosystem II which are released into lumen, also contribute in building the H+ gradient and ultimately ATP synthesis (see Fig. 11.16). This chemiosmotic mechanism of coupling electron transport with phosphorylation in photosynthesis is essentially similar to that of oxidative phosphorylation in mitochondria, (For details see Chapter 16).
The proton electrochemical gradient across the thylakoid membrane is from inside (lumen of thylakoid) to outside (stroma) while in mitochondria, this gradient across the inner mitochnodrial membrane is from outside (inter-membrane space) to inside (matrix).
(The chemiosmotic method of photophosphorylation was greatly supported by experiments of Jagendorf (1967). He suspended isolated chloroplast thylakoids in dark in a pH 4 buffer. This acidic buffer penetrated into the inner compartments of thylakoids lowering their internal pH. He added ADP and Pi to the dark suspension of chloroplast thylakoids and then suddenly raised the pH of outer medium to 8, thereby creating a large pH gradient across the membrane (the inside of thylakoids becoming more acidic than the outer medium).
As the protons (H+) moved out of the thylakoids into the outer medium, large amounts of ATP were formed in dark (with no input of light energy or electron transport). This experiment clearly showed that a pH gradient across the energy transducing membrane is a high energy state that can mediate the transduction of energy from electron transfer into the chemical energy of ATP).
(b) Cyclic Electron Transport and Cyclic Photophosphorylation:
In contrast to non-cyclic electron transport, the cyclic electron transport involves only pigment system I and takes place under conditions which exclude non-cyclic photophosphorylation. This situation is created if the activity of pigment system II is blocked.
The latter can be accomplished by the use of specific inhibitors or by using wavelengths of light greater than 680 mp. Under these conditions:
(i) Only pigment system I remains active
(ii) Photolysis of water does not take place
(iii) Blockage of non-cyclic ATP formation causes a drop in CO2 assimilation in dark reaction and
(iv) There is a consequent shortage of oxidised NADP+.
Thus, when P700 molecule is excited in pigment system I by absorbing a photon (quantum) of light, the ejected electron is captured by ferredoxin (E0’ = – 0.432 V) via FRS. From ferredoxin the electron cannot be drained off to the dark reactions of photosynthesis through oxidised NADP+ (due to the shortage of latter) and ultimately it falls back to the P700 molecule (E0‘ = 0.43 V), involving of course a number of other intermediate electron carriers of redox system.
These are probably cytochrome b6 (E0‘ = – 0.06 V), cytochrome ƒ (E0‘ = + 0.36 V) and plastocyanin (E0‘ = + 0.39 V). It is quite obvious from the E0‘ values that all these intermediate electron carriers form an electrochemical gradient of decreasing negative values which facilitates the downhill transport of electron from FRS to P700 molecule.
At two place during this electron transport i.e., between ferredoxin and cytochrome b6 and between cytochrome b6 and cytochrome ƒ there is phosphorylation of one ADP molecule to form one ATP molecule. Thus, two ATP molecules are produced in this cycle.
Because in the above electron transport system the electron which was ejected from P700 molecule is cycled back, the process has been called as cyclic electron transport and the accompanying phosphorylation as the cyclic photophosphorylation (Fig. 11.17A).
Most recent findings suggest that during cyclic electron transport, the electrons from ferredoxin (Fd) are transferred to PC and finally back to PSI through Cyt b6 f complex involving a plastoquinone. This appears to be essential for the proton gradient across the thylakoid membrane for the production of ATP from ADP and Pi in the presence of ATPase by Chemiosmotic mechanism.
Significance of Cyclic Photophosphorylation:
We have seen that during cyclic electron transport and phosphorylation photolysis of water, O2 evolution and reduction of NADP+ do not take place. It generates only ATP molecules and as such cannot drive dark reactions of photosynthesis. On the other hand non-cyclic photophosphorylation does not produce sufficient ATP in relation to NADPH to operate the dark phase of photosynthesis (Calvin Cycle).
Therefore, the deficiency of ATP molecules in non-cyclic photophosphorylation is made up by the operation of cyclic photophosphorylation instead of the former for certain periods. Secondly, cyclic photophosphorylation may be an important process in providing ATP for synthetic processes (other than photosynthesis) e.g., synthesis of starch, proteins, lipids, nucleic acids, pigments within the chloroplasts.
Thus we have seen that the light energy has been converted into chemical energy during primary photochemical reaction and is trapped in ATP and NADPH molecules. This chemical energy is finally stored in carbohydrate molecules when ATP and NADPH (i.e. the assimilatory power) are utilised in dark reaction of photosynthesis in reducing CO2 to carbohydrates.
Q-CYCLE:
Several models have been proposed by scientists to explain flow of protons and electrons through cytochrome b6ƒ complex in thylakoids in photosynthesis, but its precise mechanism is not yet fully understood. The most widely accepted model mechanism in this regard is however, known as Q-Cycle which has been illustrated in Fig. 11.17 B & C. In this mechanism, one of the two electrons from PQH2 (produced by the action of PS II) passes in a linear way towards PSI while the other electron takes a cyclic route that increases the number of protons pumped across thylakoid membrane.
As has been mentioned earlier, while describing the two pigment systems, the cytochrome b6f complex is an integral protein complex between PSII and PSI and that the two mobile electron carriers of the photosynthetic electron transport chain i.e., PQ and PC operate between PSII & cytochrome b6f complex and between cytochrome b6f complex and PSI respectively.
The cytochrome b6f complex contains two b type hemes (cytochrome b) and one c type heme (called cyt. f). It also contains a Rieske iron-sulphur protein (Fe-SR) with a unique 2Fe-2S cluster (so named after its discoverer John S. Rieske) and two quinone oxidation-reduction sites. (Cyt. b6f complex is also believed to contain an additional heme (called heme Cn), a chlorophyll, and a carotenoid whose functions are yet unknown).
(i) In the non-cyclic or linear process of this mechanism (Q-cycle), PQH2 produced by the action of PSII is oxidized near the lumenal side of the complex releasing its two electrons one each to Fe-SR and one of the two b- type cytochromes (with low reduction potential) and simultaneously expelling its two protons (2H+) into the thylakoid lumen.
From Fe-SR, the electron is transferred to oxidized PSI via PC. The reduced b-type cytochrome gives its electron to another cyt. b (with high reduction potential) which in turn reduces PQ to plastosemiquinone (PQ–). See Fig. 11.17 B.
(ii) In the cyclic process of the Q-Cycle, another PQH2 from PS II is oxidized in a similar way expelling its two protons into the thylakoid lumen and one electron going to oxidize P700 via Fe-SR, cyt. ƒ and PC. The remaining electron goes through two b-type cytochromes and reduces plastosemiquinone. The latter takes two protons (2H+) from stroma and is folly reduced to plastohydroquinone (PQH2). See Fig. 11.17 C.
Thus, for each pair of electrons which are transferred to P700, two PQH2 are oxidized to PQ state, four protons (4H+) are expelled into the thylakoid lumen and one oxidized PQ is reduced to PQH2 form (The resulting H+ gradient (high conc. of H+ inside thylakoid and low conc. of H+ outside) is believed to be the main driving force for photophosphorylation).
It is important to note that in Q-cycle described above, the two PQH2 molecules produced by the action of PSII in successive two turns contain 2 electrons each and a total of four electrons out of which only two reach PSI via cyt. b6f complex and PC.
The remaining two electrons are consumed in reducing a PQ to PQH2 along with two protons that are taken from stroma. Therefore, Q-cycle may be a mechanism to create PQ ⇋ PQH2 pool in the thylakoid membrane rather than satisfactorily explaining transport of all electrons from PS II to PS I through Cyt. b6f complex.
(A similar Q-cycle also operates in mitochondria for the passage of electrons and protons from UQH2 through complex III (Cyt bc1 complex) which accommodates the switch between the two electrons carrier PQ and one electron carriers Cyt b & Cyt c1. Cyt b6f complex of thylakoids and complex III of mitochondria are chemically analogous.)
The Dark Reaction or Blackman’s Reaction or the Path of Carbon in Photosynthesis:
The dark reaction of photosynthesis is purely enzymatic and slower than the primary photochemical reaction. It takes place in stroma portion of the chloroplast and is independent of light i.e. it can occur either in presence or in absence of light provided that assimilatory power is available.
The conversion of CO2 to carbohydrate with the help of assimilatory power (NADPH+EF & ATP) in dark reaction of photosynthesis is most thoroughly analysed part of photosynthesis. This success had been due to the availability of a long lived radioactive isotope of carbon* l4C with a half-life of 5720 years. Main credit for investigating the sequences of dark reaction in photosynthesis goes to Melvin Calvin who was rewarded by the Nobel Prize in 1961. A.A. Benson, J. Bassham and other co-workers have also contributed a lot.
By employing 14C labelled carbon dioxide (14CO2) in photosynthesis and observing the appearance of characteristic radiations in different reaction intermediates and products in different experiments, Calvin and his co-workers were able to formulate the complete metabolic path of carbon assimilation in the form of a cycle which is called as Calvin Cycle.
Essay # 8. Evidences for Existence of Light and Dark Reactions in Photosynthesis:
(i) Experiments with Intermittent Light:
The rate of photosynthesis has been found to be greater in intermittent light (i.e., light given after intervals of dark periods) than in continuous light. It indicates the existence of two stages in photosynthesis i.e., a light and another dark.
The light reaction is faster than the dark reaction. In continuous light the products of the light reaction (i.e., NADPH2 + ATP) are not consumed at the same rate in subsequent dark reaction at which they are produced in light reaction resulting in their accumulation. But with intermittent light the products of light reaction are consumed to fix CO2 to carbohydrates both during the light period and the interval of dark period. Accumulation of NADPH2 + ATP is prevented because they are not produced during dark periods.
(ii) Temperature Coefficients:
The ratio of the rate of a particular process at a certain temperature to the rate of that process exactly at 10°C lower is called as temperature coefficient and is denoted by Q10. For purely chemical processes the value of Q10 is 2 or more i.e., the rate of the process will become double or more by raising the temperature by 10°C. But for photochemical reactions Q10 is approximately one.
Blackman has observed that if the leaves were well illuminated and received an ample supply of CO2, the value of Q10 for photosynthesis was 2 or more. But in poorly illuminated leaves Q10 was 1. These results support the existence of a purely chemical reaction i.e., the dark reaction and a photochemical reaction i.e., the light reaction in photosynthesis.
(iii) Physical Separation of the Light and Dark reactions:
Experiments have been successful in separating grana and stroma portions of the choloroplasts. If the isolated grana are illuminated in presence of suitable H-acceptors and in complete absence of CO2, oxygen is released and assimilatory power is generated. The latter when supplied to stroma portion in the presence of CO2 and even in complete absence of light resulted in the production of carbohydrates. These experiments clearly indicate the existence of a light dependent reaction (localised in grana) and a light independent reaction or dark reaction (localised in stroma) in photosynthesis.
Essay # 9. Source of Oxygen Released in Photosynthesis:
Before 1930’s it was thought that O2 released in photosynthesis comes from CO2. But the idea that O2 comes from H2O and not from CO2 which is now well established was first given by Van Niel (1930-31). He observed that in certain photosynthetic bacteria such as green sulphur bacteria, oxygen is not released during photosynthesis. Such bacteria instead of utilizing H2O make use of H2S and liberate sulphur.
This prompted him to think that as sulphur comes from H2S in green sulphur bacteria in photosynthesis, similarly, oxygen must come from H2O in algae and higher plants and proposed a general formula for photosynthesis.
In the above formula A will represent sulphur in case of green sulphur bacteria while in algae and the other higher green plants it will represent oxygen.
The idea of Van Niel was greatly supported by the experiments of Hill (1937) and isotopic studies done by Ruben, Kamen and coworkers (1941).
Hill observed that if the isolated chloroplasts were illuminated in complete absence of CO2 but in presence of suitable H-acceptors (Hill’s oxidants) such as indophenols and ferric salts, oxygen was released.
4Fe3+ + 2H2O → 4Fe2+ + O2 + 4H+
Ruben et al using a heavy isotope of oxygen (O18) showed that if the photosynthesis took place in presence of H2O18 and normal CO2 the oxygen was found to be isotopically labelled.
2H2O18 + CO2 → O218 + (CH2O) + H2O
But, if normal H2O and CO218 were supplied, the O2 evolved during photosynthesis did not contain the heavy isotope.
2H2O + CO218 → O2 + (CH2O18) + H2O
Essay # 10. Factors Affecting the Photosynthesis:
The rate of photosynthesis is affected by a number of external and internal factors. The principle of limiting factors should be kept in mind while studying the effect of various factors on photosynthesis.
(A) External Factors:
1. Light:
In nature the chief source of light for photosynthesis in green plants is sun-light. Moon light has been found to be effective in marine algae. Besides these, any kind of artificial light e.g., electric light can induce photosynthesis in green plants, provided it is in the visible part of the spectrum. The light is an essential factor for photosynthesis.
It affects the rate of photosynthesis in three ways:
(i) Light Quality:
Photosynthesis in green plants takes place in the visible part of the spectrum of light. Although the light rays of longer wavelengths have lower energy than the light rays of shorter wavelengths, but owing to the heavy absorption in the red part of the spectrum by chlorophylls, maximum photosynthesis takes place in red light.
The next highest rate of photosynthesis takes place blue light while in green light it is minimum. The rate of photosynthesis is higher in white light (such as day light) than in monochromatic light (i.e., light rays of a particular colour).
(ii) Light Intensity:
Usually the rate of photosynthesis is greater in intense light than in diffused light. But certain plants require less intense light for optimum photosynthesis and grow in shady places. They are called as sciophytes. On the other hand, the plants which grow in sunny places require more intense light for optimum photosynthesis. They are called as heliophytes.
Extremely intense light may have a direct inhibitory effect on photosynthesis, a phenomenon called as solarization. During solarization photo-oxidation of cellular components including the photosynthetic apparatus takes place which may even result in their death. Secondly, in extremely intense light other factors may soon become limiting thus reducing the rate of photosynthesis.
(iii) Duration of Light:
Even a brief flash of light is enough for photosynthesis to occur. However, the rate of photosynthesis is greater in intermittent light than in continuous light. It is because in continuous light the assimilatory power accumulates and is not consumed in the dark reaction at the same rate at which it is produced in light reaction.
2. CO2:
CO2 constitutes about 0.03% (330 ppm) by volume of the atmosphere. An increase in CO2 concentration up to about 1% increases the rate of photosynthesis. But very high conc. may prove toxic and the rate of photosynthesis will go down.
3. Temperature:
Different plants have different requirement of temperature for photosynthesis. For example, photosynthesis will stop in many plants at about freezing point but in some conifers it takes place even at—35°C. Similarly, temperatures beyond 40—50°C retard photosynthesis in most of the plants but certain xerophytes like Opuntia and algae growing in hot springs carry on photosynthesis even at 55°C and 75°C respectively. Usually, an increase in temperature from 10°C to about 40°C brings about an increase in the rate of the photosynthesis. Q10 for photosynthesis is 2.
4. Water:
Water is one of the raw materials and an essential factor for photosynthesis. Usually the water rarely acts as a limiting factor for photosynthesis but the rate of photosynthesis may go down if the plants are inadequately supplied with water.
5. O2:
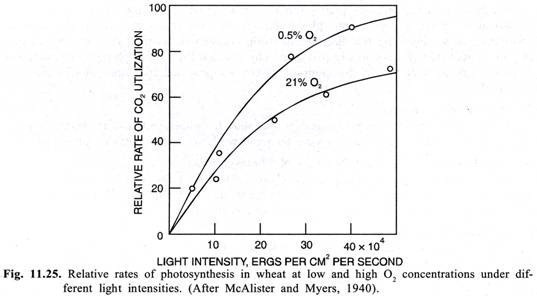
Warburg’s Effect:
Despite the photosynthetic origin of oxygen, an increase in the O2 concentration in may plants results in a decrease in the rate of photosynthesis. This phenomenon of the inhibition of photosynthesis by O2 was first discovered by a German biochemist Warburg in 1920 in green alga Chlorella and is called as Warburg’s effect. This effect was subsequently confirmed in wheat by Mc-Alister and Myers (1940) and is now known to operate in a number of other plants like soya bean etc. (C3 plants). This is not shown by plants like maize, sugarcane, sorghum etc. which are called as C4 plants.
O2 causes greatest inhibition of photosynthesis when CO2 levels are low and light levels are saturating. The inhibition can be relieved by high CO2 concentrations. Normal atmospheric concentration of O2 is about 21% which is sufficiently high to slower the rate of photosynthesis that prevails at lower O2 concentrations in plants showing Warburg’s effect (Fig. 11.25).
Because, the inhibition of the rate of photosynthesis is maximum when light levels are saturating, it was thought that the O2 is interfering with the generation of reducing power (NADPH,) or as Warburg (1920) suggested the O2 may be reoxidising a primary photochemical product and thus competing with CO2 for reducing power of this product. But the work of Ellyard & Gibbs (1969) has shown that generation of reducing power is unaffected by O2 and that this process would not be rate limiting for most environmental conditions.
There appears to be a close relation between Warburg’s effect and the process of photorespiration in plants. As will be discussed later, the substrate of photorespiration is glycolate which is synthesized from some intermediates of Calvin cycle.
In plants which show Warburg’s effect increased O2 concentrations result in diversion of these intermediates of Calvin cycle into the synthesis of glycolate thereby showing higher rate of photorespiration and consequently lower photosynthetic productivity. The plants which do not show Warburg’s effect also lack photorespiration and consequently are more efficient photosynthetically.
(B) Internal Factors:
1. Chlorophyll Content:
Chlorophyll is essential for photosynthesis. In etiolated plants and the non-green parts of variegated leaves in some plants photosynthesis does not take place. Although there are conflicting views regarding the direct relationship between chlorophyll content and the rate of photosynthesis, but theoretically it is quite obvious that the rate of photosynthesis should increase with an increase in the chlorophyll content provided the other factors are also favourable.
2. Protoplasmic Factors:
Proper hydration of the protoplasm is essential for photosynthesis. However, isolated chloroplasts are also capable of carrying on photosynthesis under suitable conditions.
3. Accumulation of the End Products of Photosynthesis:
Accumulation of carbohydrates in the photosynthesizing cells retards the rate of photosynthesis. Quick translocation of the carbohydrates or the end products of photosynthesis will have a favourable effect on the rate of photosynthesis.
4. Anatomy of Leaf:
The rate of photosynthesis is greatly influenced in any leaf or other photosynthesizing part of the plant by the anatomy of that leaf or plant part. The thickness of the cuticle and epidermis, structure and distribution of stomata, distribution and relative proportion of chlorophyllous and non-chlorophyllous mesophyll tissues and structure and distribution of the vascular tissue all influence the rate of photosynthesis.
The effect of the internal structure of the leaves or other photosynthesizing parts of the plant on the rate of the photosynthesis results chiefly due to its influences on the entrance of the CO2, intensity of light penetrating to chlorophyllous cells, maintenance of the turgidity of such cells and the translocation of the soluble sugars (i.e., the end products of photosynthesis) out of these cells through vascular tissue.
5. Microstructure of Chloroplasts:
The microstructure of the chloroplasts may also influence the rate of photosynthesis or even determine its course. It is now well known that the plants which show Hatch-Slack pathway contain two types of chloroplasts in contrast to the C3 plants which have only one type of chloroplasts.