In this essay we will discuss about Viruses. After reading this essay you will learn about:- 1. Meaning of Viruses 2. Origin of Viruses 3. Features 4. Early Development of Virology 5. Classification 6. Structural Organisation 7. Chemistry 8. Assay 9. Biological Status 10. Cultivation 11. Transmission.
Contents:
- Essay on the Meaning of Viruses
- Essay on the Origin of Viruses
- Essay on the Distinguishing Features of Viruses
- Essay on the Early Development of Virology
- Essay on the Classification of Viruses
- Essay on the Structural Organization (Symmetry) of Viruses
- Essay on the Chemistry (Structural Components) of Viruses
- Essay on the Assay of Viruses
- Essay on the Biological Status (Nature) of Viruses
- Essay on the Cultivation of Viruses
- Essay on the Transmission of Viruses
Contents
- Essay # 1. Meaning of Viruses:
- Essay # 2. Origin of Viruses:
- Essay # 3. Distinguishing Features of Virus:
- Essay # 4. Early Development of Virology:
- Essay # 5. Classification of Viruses:
- Essay # 6. Structural Organization (Symmetry) of Viruses:
- Essay # 7. Chemistry (Structural Components) of Viruses:
- Essay # 8. Assay of Viruses:
- Essay # 9. Biological Status (Nature) of Viruses:
- Essay # 10. Cultivation of Viruses:
- Essay # 11. Transmission of Viruses:
Essay # 1. Meaning of Viruses:
Viruses can be defined as “non-cellular, sub-microscopic, obligatory intracellular parasites composed of a proteinaceous covering around central nucleic acid (either DNA or RNA) and capable of self-replication within the living host cells.”
Viruses are a unique class of infectious agents truely distinctive by their simple organization and mechanism of replication. They are among the most numerous “microorganisms” on our planet and infact all types of cellular organisms. The usual English plural of “virus” is “viruses” although its Latin plural “vira” may also be used. The study of viruses is called virology and the specialists in the field of virology are called virologists.
Viruses exist in two states, extracellular and intracellular. In the extracellular state, a virus is a minute particle containing nucleic acid surrounded by protein and occasionally, depending on the specific virus, containing other macromolecular components. In this typical extracellular form, the virus partical, also called virion is metabolically inert and does not carry out respiratory or biosynthetic functions.
The virion is the structure by which the virus genome is carried from the cell in which it has been produced and another cell where the virus nucleic acid can be introduced. Once in the new cell, the intracellular state is initiated. In the intracellular state, virus replication occurs; new copies of the viral genome are produced and the components that make up the virus coat are synthesized.
Since viruses consist of proteins and nucleic acids, they are actually nucleoproteins (also called nucleocapsid). The protein coat of the virus is called capsid, which is in contact with and encloses the nucleic acid. Capsomers are the individual protein units, which comprise the capsid of viruses. A strongly pathogenic virus is called virulent. Vector indicates an organism, usually an insect that carries and/or transmits disease causing viruses.
Aster yellow virus was the first plant virus shown to multiply in insect vectors (i.e., propagative virus). Satellite tobacco necrosis virus (STNV) is the smallest known plant virus. Pox virus is the largest known animal virus. First successful vaccination against small pox was carried out by E. Jenner. First successful isolation of a virus, the bacteriophage-WLL from the bacterium E. coli, was done by M. Schlesinger (1933).
Essay # 2. Origin of Viruses:
Three theories have been put forward to explain the origin of viruses.
These theories highly speculative and are as follows:
(i) Survivors of Pre-Cellular First Living Inhabitants of the Earth:
This theory intimately rests on the theory of origin of life on Earth. Life, according to this theory, originated from simple inorganic compounds by a slow biochemical evolution of “ordinary” chemical reactions spread over millions and millions of years.
It is speculated that during the course of origin of life on Earth somewhere at the stage when complex chemical molecules united to form still more complex molecules which could mate with still other metastable molecules till a relatively large molecule (like nucleoproteins) capable of growth and division, a simple virus or a protovirus may have originated. This theory, however, enjoys some insurmountable objections.
Present day viruses are all obligate parasites and it is difficult to conceive of their origin before the origin of their hosts (cells) which are at a far higher scale of evolution.
Viruses use the same genetic code as cellular organisms and depend solely and entirely on ribosomes, transfer RNAs and enzymes of the host cell for protein biosynthesis. Moreover, viral nucleic acid has the same properties and the same mode of replication as the nucleic acid of cellular organisms.
(ii) Regression from More Highly Evolved Free-Living Microorganisms/Cells:
Viruses are considered to have originated by retrogressive evolution from free-living cells, according to this theory. A parasite evolves retrogressiveIy as it takes the readymade metabolites from its host instead of synthesizing them himself.
If the parasite continues to evolve retrogressively then, to save labour and energy it would slowly loss some of its physiological, morphological and even genetical functions that become super numerary in its new ecological niche and new mode of biological existence. A parasite would, therefore get regressed to a much simpler organism.
Obligate intracellular parasitism is the most specialized type of parasitism and such a parasite would ultimately possess only the bare minimum and this minimum is the possession of a nucleic acid (to ensure genetic continuity) enclosed within a protein shell (to ensure safety of the nucleic acid).
Shedding of all unnecessary morphological, physiological and genetical materials would necessarily reduce the size. A formerly free-living organism would thus be transformed into a virus. Many virologists support the theory of retrogressive origin of viruses but the same is opposed also by others.
(iii) Derived from Normal Constituents of the Cell:
An eukaryotic cell possesses organelles like chloroplasts and mitochondria which are self-replicatine semi- autonomous structures and reproduce their like. Chloroplasts and mitochondria increase in size and then divide while kinetosome is synthesized near a pre-existing one by assembly from the tubular materials.
Besides they possess DNA which is functional and possesses its own mutational history, codes for the synthesis of mRNA and also presumably for protein. Mitochondria mutate to non-functional forms in Neurospora and yeasts, while chloroplasts mutate to undeveloped, colourless proplastids in algae and higher plants.
These mutations are based on genetic changes. Cells also contain certain organelles that exceptionally undergo autonomous unrestricted replications; examples are the centrioles in Marsilea and sperms and nuclear genes in the amphibian oocytes.
Viruses resemble above mentioned cellular organelles in chemical composition, possession of nucleic acid, genetic continuity of genome, independent mutational history, capacity to replicate independently under their own genetic control and also under the overall regulatory control of and within the premises of the host cell.
Many of the cellular organelles or factors possess some of the distinctive characters of viruses, or more specifically, of viral genetic determinant (reproductive independence, evolutionary independence, independent cell to cell transfer and infectivity and pathogenicity) while others could be conferred on them by a specific arrangement of nucleotides.
Viruses could, therefore, be derived from any or several of these cellular components and it is possible that different viruses have originated differently.
Some of the possibilities are given below:
1. Primordial self-replicating molecules may have mutated to regain the capacity to enter (‘infect’) the cell and then integrate with it. Such a molecule would be a ‘virus’.
2. Some plasmids or even chromosomal segments may have evolved by merging of some primitive self- replicating molecules with the cellular genome. If this is true then it is conceivable that some of the genes or groups of genes may revert to their ancestral habit and may have regained/evolved the genetic independence and independent transfer of this genetic material. This would result in the ‘virus’.
3. Some genes of the cell could have escaped of the control mechanisms of the cell and may have acquired the capacity of autonomous replication independent of the division of the cell and capacity of independent transfer. Integration of these genes with the host genome would give us a prophage.
Origin of bacteriophage from such prophage DNA has been outlined by Lindegren in 1962. It is suggested by some that bacteriophages containing DNA may have evolved from a number of genetic transfer elements (like F factor, bacteriocinogenic factor, etc.) occurring in the prokaryotic cells.
4. DNA viruses of eukaryotic cells may have originated from the functional DNA of cellular organelles (e.g., mitochondria and chloroplast) rather than for nuclear DNA.
5. Origin of DNA plant viruses is somewhat more difficult to understand since there is no definite information with respect to the integration of plant viral nucleic acid into the genome of the plant cell. There is, however, some suggestive evidence in this connection. Some experimental evidence suggests that cut surfaces of barley seeds take up the DNA of the bacterium Micrococcus lysodeikticus and that it integrates into nuclear DNA of barley and replicates.
In short, therefore, viruses may have originated from cell constituents which escape the control mechanisms of the cell, regained/developed the capacity of autonomous self-replication and ability to mediate their own independent cell to cell transfer and could enter or infect cells to which they did not belong.
Essay # 3. Distinguishing Features of Virus:
(i) All viruses are obligate parasites,
(ii) They multiply only within their living hosts cells and remain metabolically inert outside the host cell,
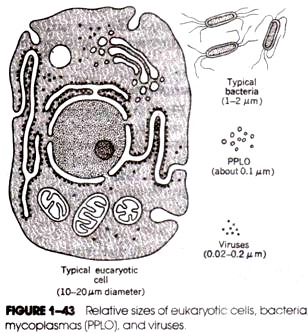
(iii) They are ultramicroscopic and can only be viewed with electron microscope (the smallest known virus is merely 0.002 µm in diameter, while the largest ones are typically about 0.8 µm in diameter),
(iv) Viruses are, actually, nucleoproteins. The proteinaceous coat (capsid) surrounds the nucleic acid, which forms the central core of the virus particles,
(v) The viral genetic material, the nucleic acid, may be either DNA or RNA. The two nucleic acids are never present in a given virus,
(vi) These are the nucleic acids of the viruses which are infectious, and not the protein coat,
(vii) Viruses are usually so minute that they can easily pass through a filter, which can hold back even the smallest bacteria,
(viii) Viruses are easily transmitted from infected host to the healthy ones through various agencies,
(ix) Viruses are so effective that even their smallest amount can cause infection on the host successfully,
(x) Viruses can easily be crystallized,
(xi) Viruses are considered to be host-specific and represent obligate parasitism even at genetic level, and
(xii) Since the viruses have no metabolic activities of their own and utilize the metabolism of host cells, antibiotics have no effect on them.
Essay # 4. Early Development of Virology:
Ancient Acquaintance with Viral Diseases:
Literature reveals that ancient people were well acquainted with today’s viral diseases such as small pox, cowpox, rabies, measles, etc. without any knowledge of the nature of the diseases.
There is some evidence that the Roman Empire was severely weakened and declined due the great epidemics of measles and smallpox diseases that appeared during A.D. 150 to 180 and A.D. 251 to 266. Aztec Empire in Mexico was captured by Hernan Cortes, a Spanish, in the 1520s. It is considered that this conquest was made possible by smallpox epidemic that ravaged Mexico city.
Early Prevention of Viral Diseases:
Prevention of viral diseases by immunization progressed years before the discovery of viruses. This practice was used in Asia for centuries to produce immunity to smallpox before it was introduced in England in 1718 by Lady Mary Montagu, wife of English ambassador in Turkey.
She observed that Turkish women inoculated their children against smallpox by material taken from a pustule of an infected person. The material was scratched into the skin of the children to be immunized. In most cases this resulted in a mild case of smallpox without he scarring that was common in naturally acquired cases.
The immunization against smallpox was practiced without any explanation of how or why it worked. Later during the end of the century an English country doctor Edward Jenner, stimulated by a girl’s claim that she could not catch smallpox because she had had cowpox, reported to Royal Society in London the value of immunization with cowpox as a means of protecting against smallpox; a clear case of vaccination.
This he did on the basis of the fact that when he inoculated a 8-year old boy, James Phipps, with cowpox content, the boy escaped from smallpox infection.
Jenner then began inoculating humans with material from cowpox lesions, and published the results of 23 successful vaccinations in 1798. Jenner’s explanation regarding cowpox vaccination against smallpox established the scientific credibility of vaccination to prevent disease and was accepted by the scientists and physicians of the time.
Jenner, therefore, is credited to have developed first vaccine from cowpox (Latin name Vaccinia). Later on, Louis Pasteur developed vaccines (attenuated microorganisms) against chicken cholera and anthrax in 1880, and against rabies in 1885.
Discovery of Viruses:
The tobacco crop in Holland was struck by a severe disease around 1870. Adolf Mayer, Director of Agricultural Experimental Station, Wageningen, began his studies on this disease about 1880 and published his results in 1886.
Mayer christened the disease as “MOSAIKKRANKHEIT” (mosaic-like), from the mosaiclike pattern on leaves of diseased plants and succeeded in reproducing the disease by infecting juice extracted from infected tobacco leaves onto healthy ones; he could not succeed in identifying the real agent that caused the disease.
However, Mayer’s contribution will always be remembered as he was the first person who put first step forward in the development of a new discipline later recognised as ‘Virology’.
The development of the porcelain bacterial filter in 1884 by Charles Chamberland, one of the Pasteur’s collaborators and the discoverer of autoclave, made possible the discovery of what are now called viruses.
Chamberland’s filter was first used in the study of tobacco mosaic disease in the year 1892 when D. Ivanowski first successfully experimentally demonstrated that the tobacco mosaic disease has been caused by agents which successfully passed the Chamberland-filter that retains even the smallest bacteria.
It was an important clue, but contrary to his experimental result and despite his inability to isolate any bacterium, Ivanowski still maintained that either the ‘pathogenic bacterium’ somehow passed through the filter or a ‘toxin’ secreted by them passed through the filter and made the filtrate infectious.
Within six years after the experiments of Ivanowski, M. Beijerinck (1898) confirmed by repeating the same experiments and found that the tobacco mosaic disease was caused not by any pathogenic bacteria or toxin but rather by some new type of pathogenic agents which he called “contagium vivum fluidum” (infectious living fluid) and referred subsequently to it as a “virus” (poison).
He observed that the viruses multiply only inside the living cell, but can survive for long periods in a dried state.
At the same time F. Loeffler and Paul Frosch in Germany demonstrated that the hoof and mouth disease of cattle was also caused by a filterable virus rather than by a toxin. W. Reed (1900) studied the yellow fever disease spreading in Cuba and demonstrated that this human disease was due to a filterable virus that was transmitted by mosquitoes.
Mosquito control shortly reduced the severity of the yellow fever disease. Thus by the beginning of 20th century, it has been established with certainty that filterable viruses were independent intities different from bacteria and were able to cause diseases in plants, animals, and humans.
Post-Discovery Contributions:
After the discovery of viruses as independent intities existing in the biological world, certain important developments were made in the field of virology.
In 1908, V. Ellerman and O. Bang reported lukemia as a viral disease. Peyton Rous reported in 1911 that a virus was responsible for a malignant muscle tumour in chickens. Discovery of bacteriophages, the viruses that attacked bacteria, was another milestone in the development of virology.
The first published observation suggesting that the bacteria themselves also could be attacked by viruses was made in 1915 by F.W. Twort. Twort observed that the colonies of micrococci and intestinal bacilli bacteria were lysed and the lytic effect spread from one colony to the other. He speculated that the lysis of bacterial colonies would have been due to a virus.
Twort did not follow up these observations and it remained for Felix d’Herelle to establish decisively the existence of bacterial viruses. D’Herelle isolated bacterial viruses from patients with dysentery, probably caused by Spigella dysenteriae.
He observed that when a virus suspension was spread on a layer of bacteria growing on agar, clear circular areas containing viruses and lysed bacterial cells appeared. D’Herelle demonstrated that bacterial viruses could reproduce only in live bacterial cells, and named these viruses ‘bacteriophages’ because could eat bacterial cells creating clear areas (the plaques) in bacterial “lawns”.
Establishment of chemical nature of viruses was a very significant contribution in the field of growing virology. W.M. Stanley (1935) first crystallized the tobacco mosaic virus (TMV) and found it to be largely or completely protein.
Shortly later, F.C. Bawden and N.W. Pirie separated tobacco mosaic virus particle into protein and nucleic acid. Thus by the late 1930s it was established with certainty that the viruses were made up of nucleic acids and proteins, and could reproduce only in living cells.
Essay # 5. Classification of Viruses:
Following is the most adopted modern classification of viruses primarily based on virus morphology, physical and chemical nature of virion constituents and genetic relatedness. Only selected groups of viruses are described in brief accompanied with illustrations to provide a general idea of the morphology of each group.
(i) The Viruses that Multiply only in Plants:
1. Caulimoviridae:
Open circular dsDNA with single-strand discontinuities like the hepadnavirus. Mol. wt. 4 to 5 x 106. Isometric 50 nm particles; some are bacilliform. Contains reverse transcriptase. Aphid vectors. Includes cauliflower mosaic virus.
2. Gerniiniviridae:
Circular single-stranded DNA of mol. wt. 0.7 to 0.8 x 106. Two incomplete icosahedral particles joined as a pair. ~18 x 30 nm, and usually found in the nucleus. One or two molecules of DNA per pair of particles. Persistent in whitefly or leafhopper vectors.
3. Luteoviridae:
Single-stranded, positive-sense RNA of mol. wt. 2 x 106. Isometric 25 to 30 nm particle. Persistent retention by aphid vectors. Includes barley yellow dwarf virus.
4. Tombusviridea:
Single-stranded, linear, positive-sense RNA of mol. Wt. 1.5 x 106. Not polyadenylated. Particle 32 to 35 nm in diameter. Cytoplasmic, nuclear, and sometimes mitochondrial location. Transmitted through the soil and by mechanical inoculation, contact, and seeds. Includes tobacco necrosis virus and tomato bushy stunt virus.
5. Bromoviridae:
Three single-stranded, linear, positive-sense RNAs. Icosahedral particles 26 to 35 nm diameter and bacilliform particles (18 to 26 nm x 30 to 85 nm). Infectivity requires the three RNAs. Each is in a different particle. Assembly in cytoplasm. Some with a beetle vector. Includes brome mosaic virus, cucumber mosaic virus, and alfalfa mosaic virus.
6. Comoviridae:
Two 28 to 30 nm non-enveloped particles containing single-stranded, linear, positive-sense RNA of mol. wt. 2.4 or 1.4 x 106. Two coat polypeptides encoded by the smaller RNA. Both RNAs needed for infectivity. Cytoplasmic. Transmitted by beetles or seed. Includes cowpea mosaic virus and tobacco ring spot virus.
7. Tobamovirus (Tobacco mosaic virus group):
Single-stranded, linear, positive-sense RNA of mol. wt. 2 x 106 Rigid c .indrical particles 300 x 18 nm. Transmitted mechanically or by seed.
8. Potyviridae:
Single-stranded, linear, positive-sense RNA of mol. wt. 3.0 to 3.5 x 106. Particle is a flexuous rod with helical symmetry, usually 650 to 900 x 11 to 15 nm. Cytoplasmic but some have nuclear inclusions. Non-persistent in aphid vectors. Includes potato virus y.
(ii) Viruses that Multiply only in Bacteria (Bacterial Viruses):
1. Family: Myoviridae (Phages with contractile tails):
Linear double-stranded DNA of mol. wt. 120 x 106. Head isometric or elongated, 110 x 80 nm; complex contractile tail 113 x 16 nm. Tail has collar, base plate, spikes, and fibres. Includes T2, T4, T6, PBS1, SP8, SP50, P2, Mu.
2. Family: Siphoviridae (Phages with long, non-contractile tails):
Linear double-stranded DNA of mol. wt. 33 x 106. Head 60 nm diameter; long non-contractile tail up to 570 nm. No breakdown of host DNA. Includes 1, χ (chi), and φ80.
3. Family: Podoviridae (Phages with short tails):
Linear double-stranded DNA of mol. wt. 25 x 106. Head 60 nm diameter. Short (17 x 8 nm) tail with 6 short fibers. Includes T7 and P22.
4. Family: Tectiviridae (Phages with double capsids):
Linear double-stranded DNA of mol. wt. 10 x 106 in 63 nm particles. Contains internal lipid. Double capsid with a rigid outer shell and flexible inner coat. After injection of DNA a tail structure of about 60 nm appears. PRD1, Bam35.
5. Family: Plasmaviridae (Pleomorphic phages):
Circular double-stranded DNA of 8 x 106 mol wt in lipid-containing envelope. 50 to 125 nm particle with small dense core. Slightly pleomorphic. Formed by budding. Infects mycoplasmas.
6. Family: Corticoviridae (PM2 phage group):
Circular double-stranded DNA of mol. wt. 6 x 106. Isometric 60 nm particle, lipid between protein shells, no envelope, no tail. Spikes at vertices. Infects Pseudomonas.
7. Family: Microviridae (Isometric phages with ssDNA):
Circular single-stranded DNA of mol. wt. 1.6 to 1.7 x 106. Icosahedron (25 to 27 nm) with knobs on 12 vertices. No envelope. Includes φX174 and G4.
8. Family: Inoviridae (Rod-shaped phages):
Helical, filamentous or rod-shaped phages with a circular, single-stranded, positive-sense DNA. No host lysis.
Genera:
Inovirus: DNA of 1.9 to 2.7 x 106, long flexible filamentous particle 760 to 1,950 x 6 to 8 nm. Host bacteria not lysed. Includes M13 and fd phages.
Plectovirus: Mycoplasma virus type 1 phages. DNA 2.5 to 5.2 x 106 mol. wt. Short rods of 85 to 280 x 14 nm.
9. Family: Cystoviridae (φ6 phages group):
Three molecules of linear, double-stranded RNA of mol. wt. 2.3, 3.1, and 5.0 x 106. Isometric 75 nm particle with lipid envelope. Infects Pseudomonas.
10. Family: Leviviridae (Single-stranded RNA phages):
Linear, single-stranded, positive-sense RNA of mol. wt. 1.2 x 106. Icosahedral capsid is 24 nm.
Genera:
Levivirus—coliphage MS2 group
Allolevivirus—coliphage Qβ group.
(iii) Viruses that Multiply only in Algae, Fungi and Protozoa:
1. Family: Totiviridae:
Isometric 40 to 43 nm particles with a genome consisting of a single molecule of double-stranded RNA of mol. wt. 3.3 to 4.2 x 106. One major capsid protein. Single shell. Transcriptase present. Cytoplasmic.
Genus:
Totivirus (Saccharomyces cerevisiae virus LA)
2. Family: Phycodnaviridae:
Large polyhedral 130 to 200 nm diameter particles containing linear, double- stranded DNA of mol. wt. 150 to 210 x 106. Non-enveloped. Contain lipid in shell. Infect Paramecium and Chlorella sp.
(iv) Viruses that Multiply in Vertebrates and Other Hosts:
1. Family: Iridoviridae:
Icosahedral particle (125 to 300 nm) consisting of a spherical nucleocapsid surrounded by lipid modified by protein subunits. Envelope may be present but is not required for infectivity. Double-stranded DNA of mol. wt. 100 to 250 x 106, which is circulatory permuted with direct terminal repeats. Contain several enzymes. Transcription and DNA synthesis are nuclear. mRNAs have no polyA tails. Cytoplasmic.
Selected genera:
Iridovirus (small 120 nm blue iridescent viruses of insects).
Chloriridovirus (Large 180 nm iridescent viruses of insects)
Ranavirus (frog virus group)
Lymphocystivirus (viruses of fish)
2. Family: Poxviridae:
Double-stranded DNA of mol. wt. 85 to 240 x 106 with inverted terminal repeats. Largest viruses 170 to 260 x 300 to 450 nm. Complex structure composed of several, layers and includes lipid.
Core contains all enzymes required for mRNA synthesis. Cytoplasmic multiplication.
Selected genera:
3. Family: Parvoviridae:
Single-stranded DNA of mol. wt. 1.5 to 2.0 x 106. Particle is an 18 to 22 nm icosahedron. Contains no enzymes. Multiplication has a nuclear stage.
Genera:
Parvovirus—viruses of vertebrates including humans. Virions mostly DNA.
Dependovirus—adeno-associated virus. Infects vertebrates. Particles contain either + DNA or -DNA, which forms a double strand upon extraction. Require helper adenovirus or herpes-virus for efficient replication.
Densovirus—viruses of insects. Virions -DNA or +DNA. Helper not required.
4. Family: Reoviridae:
Ten to twelve segments of double-stranded RNA of total mol. wt. 12 to 20 x 106. Particle is a 60 to 80 nm icosahedron. Has an isometric nucleocapsid with transcriptase activity. Cytoplasmic multiplication.
Selected genera:
Orthoreovirus—of vertebrates
Orbivirus—of vertebrates, but also multiply in insects.
Coltivirus—of vertebrates and ticks
Rotavirus—of vertebrates
Cypovirus—cytoplasmic polyhedrosis viruses of insects
Phytoreovirus—wound tumor virus.
5. Family: Picornaviridae:
Single-stranded, positive-sense RNA of mol. wt. 2.5 x 106. Particle is 30 nm and icosahedral. Multiplication is cytoplasmic.
Genera:
Enterovirus (acid-resistant, primarily viruses of gastrointestinal tract)
Hepatrovirus (hepatitis A virus group)
Rhinovirus (acid-labile, mainly viruses of upper respiratory tract)
Aphthovirus (foot-and-mouth disease virus)
Cardiovirus (EMC virus of mice)
Also various viruses of insects.
6. Family: Togaviridae:
Single-stranded, positive-sense RNA of mol. wt. 4 x 106. Enveloped particles 60 to 70 nm diameter contain an icosahedral nucleocapsid. Hem-agglutinate. Cytoplasmic, budding from plasma membrane. Have a sub-genomic mRNA.
Genera:
Alphavirus (arboviruses, e.g., Semliki Forest and Sindbis viruses)
Rubivirus (rubella virus)
7. Family: Flaviviridae:
Single-stranded RNA of mol. wt. ~ 4 x 106. Enveloped particles 40 to 60 nm diameter. Differ from Alphaviridae by presence of a matrix protein, the lack of intracellular sub-genomic mRNAs, and budding from the endoplasmic reticulum. Hemagglutinate. Cytoplasmic.
Genera:
Flavivirus (arboviruses, e.g., yellow fever virus)
Pestivirus (e.g., hog cholera)
Hepatitis C virus group.
8. Family: Rhabdoviridae:
Single-stranded, negative-sense RNA of mol. wt. 3.5 to 4.6 x 106 complementary to mRNA. The bullet-shaped or bacilliform (100 to 430 x 70 nm) particle is enveloped with 5 to 10 nm spikes. Inside is a helical nucleocapsid with transcriptase activity. Cytoplasmic, budding from plasma membrane. Some are orboviruses.
Genera:
Vesiculovirus (vesicular stomatitis virus group) viruses of vertebrates and insects.
Lyssavirus (viruses of vertebrates, e.g., rabies, and of insects, e.g., sigma).
Plant viruses (e.g., lettuce necrotic yellows, potato yellow dwarf, and many others).
9. Family: Bunyaviridae:
Three segments (large, medium, and small) of single-stranded RNA of total mol. wt. 6 x 106. Enveloped 100 nm particles with spikes and three internal ribonucieoprotein filaments 2 nm wide. Cytoplasmic, budding from the Golgi apparatus. Arthropod-transmitted except Hantavirus genus.
Selected genera:
Bunyavirus (Bunyamwera and 150 or so related viruses).
Hantavirus (Korean hemorrhagic fever or Hantaan virus) not arboviruses.
Tospovirus (tomato spotted wilt group).
(v) Viruses that Multiply only in Vertebrates:
1. Family: Herpesviridae:
Double-stranded DNA of mol. wt. 80 to 150 x 106. Particle is a 100 to 110 nm icosahedon enclosed in a lipid envelope (120 to 200 nm virion diameter). Buds from nuclear membrane. Latency for the lifetime of the host is common.
Subfamily:
Alphalierpesvirinae
Genera:
Simplexvirus—huamn (alpha) herpesvirus 1 and 2 (herpes simplex virus types 1 and 2)
Varicellovirus—human (alpha) herpesvirus 3 (varicella-zoster virus).
Subfamily: Betaherpesvirinae (cytomegaloviruses)
Genera:
Cytomegalovirus—human cytomegalovirus group e.g., human (beta) herpesvirus 5 (human cytomegalovirus)
Muromegalovirus—mouse cytomegalovirus group
Subfamily:
Gammaherpesvirinae (lymphoproliferative virus group)
Genera:
Lymphocryptovirus—e.g., human (gamma) herpesvirus 4 (Epstein-Barr virus).
2. Family: Adenoviridae:
Double-stranded DNA of mol. wt. 20 to 30 x 106. Particle is a 70 to 90 nm icosahedron, which replicates and is assembled in the nucleus.
Genera:
Mastadenovirus (adenoviruses of mammals)
Aviadenovirus (adenoviruses of birds)
3. Family: Polyomaviridae:
Double-stranded circular DNA. Non-enveloped, icosahedral particles are 40 nm in diameter, have 72 capsomers in a skewed arrangement, and are assembled in the nucleus. Oncogenic.
Genus:
Polyomavirus is found in rodents, humans, and other primates; DNA 3 x 106 mol. wt., includes simian virus 40 (SV-40) and polyomavirus itself.
4. Family: Papillomaviridae:
Double-stranded circular DNA. Non-enveloped, icosahedral particles are 55 nm in diameter, have 72 capsomers in a skewed arrangement, and arc assembled in the nucleus. Oncogenic.
Genus:
Papillomavirus produces warts and papillomas in several mammalian species; DNA 5 x 106 mol. wt., includes human papillomavirus (HPV).
5. Family: Hepadnaviridae:
One complete DNA minus strand of mol. wt. 1 x 106 with a 5′ terminal protein. DNA is circularized by an incomplete plus strand of variable length (50 to 100%), which overlaps the 3′ and 5′ termini of DNA minus. There is a 40-48 nm enveloped particle containing a core with DNA polymerase and protein kinase activities. Reverse transcriptase is involved in virus reproduction.
Includes hepatitis B of humans. Pekin duck hepatitis, beechy ground squirrel hepatitis, and woodchuck hepatitis viruses HBV is strongly associated with liver cancer.
Genus:
Orthohepadnavirus—hepatitis B virus.
6. Family: Caliciviridae:
Single-stranded, positive-sense RNA of mol. wt. 2.7 x 106. Icosahedral 37nm particle with calixlike (cup-shaped) surface depressions.
Genus:
Calicivirus (vesicular exanthema of swine virus. Norwalk virus, possibly hepatitis E virus).
7. Family: Arenaviridae:
Two (a large and a small) segments of single-stranded RNA of mol. wt. 3 x 106 and 1.3 x 106. Latter RNA is ambisense. Enveloped 50 to 300 nm particles with spikes. Contain ribosomes which have no known function. Cytoplasmic multiplication; buds from plasma membrane.
Genus:
Arenavirus (lymphocytic choriomeningitis virus and related viruses—e.g., Lassa, Junin, and Macupo viruses).
8. Family: Paramyxoviridae:
Single-stranded RNA of mol. wt. 5 to 7 x 106. Enveloped 150 nm particles have spikes and contain a helical nucleocapsid 12 to 17 nm in diameter with transcriptase activity. Filamentous forms common. Cytoplasmic, budding from plasma membrane. Airborne transmission.
Subfamily: Paramyxovirinae:
Genera:
Rubulavirus (mumps, Newcastle disease virus, parainfluenza virus). Only this genus has a neuraminidase activity which is on the same protein (HN) as the hemagglutination activity.
Morbillivirus (measles virus, canine distemper) Hemagglutinate.
Subfamily: Pneumovirinae:
Pneumovirus (respiratory syncytial virus group).
9. Family: Orthomyxoviridae:
Eight segments of single-stranded RNA of total mol. wt. 4 x 106. Enveloped 100 nm particles have spikes and contain a helical nucleocapsid 9 nm in diameter with transcriptase activity.
Only A and B virions have separate hem-agglutinin and neuraminidase proteins. Multiplication requires the nucleus. RNA segments in a mixed infection readily assort to form genetically stable hybrids within a virus. Buds from plasma membrane.
Genera:
Influenza virus A and B
Influenza C virus. Has seven RNA segments and a receptor-destroying activity (a sialic acid-O- acetyl esterase), which is on the hemagglutinin protein.
10. Family: Filoviridae:
Long filamentous particles 800 to 900 (sometimes 14,000) x 80 nm with helical nucleocapsid of 50 nm diameter. Linear, single-stranded, negative-sense RNA of mol. wt. 4.2 x 106. Buds from plasma membrane. Contains Marburg and Ebola viruses, which are highly pathogenic for humans. Transmitted by contact.
Genus:
Flovirus—Marburg and Ebola viruses.
11. Family: Retroviridae:
Single-stranded “diploid” RNA with unique sequence having a mol. wt. 1 to 3 x 106. Enveloped 80-100 nm particles with a core containing a helical nucleoprotein. Contains RNA-dependent DNA polymerase (reverse transcriptase). The DNA provirus is nuclear.
Selected genera:
MLV-related viruses (mammalian type C retrovirus group)—murine leukemia virus.
Spumavirus—human foamy virus.
HTLV-BLV group—human T-cell lymphotrophic viruses types 1 and 2.
Lentivirus—human immunodeficiency viruses types 1 and 2; simian immunodeficiency virus, visna virus.
(vi) Viruses that Multiply only in Invertebrates:
1. Family: Baculoviridae:
Double-stranded circular DNA of mol. wt. 60 to 110 x 106. Bacilliform particles 30 to 60 nm x 250 to 300 nm with an outer membrane. May be occluded in a protein inclusion body containing usually one particle (granulosis viruses, upper illustration) or in a polyhedra containing many particles (polyhedrosis viruses, lower illustration).
Selected genera:
Nuclear polyhedrosis virus Granulosis viruses Nonoccluded baculoviruses
2. Family: Tetraviridae:
Single-stranded RNA of mol. wt. 1.8 x 106 in a 35 nm particle. T = 4 (whereas in Picornaviridae T = 1). All isolated from Lepidoptera. No infection of cultured cells. Nudurelia β virus.
Essay # 6. Structural Organization (Symmetry) of Viruses:
Viruses, so far studied, usually fall into three main symmetry groups on the basis of electron microscopic studies. These groups are: icosahedral (or cubic), helical and complex.
(i) Icosahedral (or Cubic) Symmetry:
Many of the viruses (e.g., herpes virus, polio virus, polyomavirus, adenovirus, φX174 bacteriophage, turnip yellow mosaic virus) have icosahedral symmetry (Fig. 11.1).
They resemble small crystals and appear approximately spherical in electron mocrographs, and their capsomeres (the building blocks of the protein coat) are arranged to form an icosahedron. An icosahedron is a regular polyhedron with 20 faces formed by equilateral triangles, and 12 intersecting corners.
(ii) Helical Symmetry:
Some viruses (e.g., TMV, bacteriophage M13, influenza virus, mumps virus, measles virus, rabies virus) resemble long rods and show helical symmetry (Fig. 11.2). In these viruses the capsomeres are arranged in a helix around a single rotational axis. The capsomeres curve into a helix because they are thicker at one end than the other.
The helical capsids may be naked (e.g., TMV) or surrounded by a loose membranous envelop (e.g., influenza virus, mumps virus). Plant viruses with helical symmetry usually appear rod-shaped in electron micrographs.
(iii) Complex Symmetry:
This groups of viruses shows complex or uncertain symmetries that result in complicated morphology (Fig. 11.3).
Viruses of this group can be divided into two subgroups as follows:
(i) That illustrated by most of the bacteriophages that have a head of the shape of bipyramidal hexagonal prism attached to a tail consisting of a helical contractile sheath surrounding a central hollow core, and
(ii) That illustrated by pox viruses that do not possess clearly identifiable capsids but have several coats around the nucleic acid.
Essay # 7. Chemistry (Structural Components) of Viruses:
Viruses in extracellular state are called virions or virus particles. Virions represent the complete assembly of the infectious virus and vary widely in size ranging from 0.02 to 0.3 nm. A virion consists of a nucleic acid core surrounded by a protein coat (called capsid).
The complete complex of nucleic acid and protein is packaged in nucleocapsid (nucleoprotein) that forms the virus particle. Such virions are called naked viruses. Few viruses have more complex structure outside the nucleocapsid called envelop. Such virions are called enveloped viruses in which nucleocapsid is enclosed in a membrane.
(i) Viral Nucleic Acids (Viral Genetic Material):
We all know that all cells have double-stranded DNA as their genetic material. But, in contrast, all viruses possess either DNA or RNA as their genetic material, and it can be either single-stranded or double-stranded. However there is a third group of viruses that use both DNA and RNA as their genetic material but at different stages of their reproductive cycle (Fig. 11.4).
This third group is examplified by retroviruses which contain RNA as genetic material but replicate through a DNA intermediate, and hepadnaviruses (human hepatitis B virus) which contains DNA as genetic material but uses RNA intermediate in replication.
Despite the diversity in genetic material, however, all viruses obey the central dogma of molecular biology i.e. all genetic information flows from nucleic acid to protein. In addition, all viruses use the cell’s translational machinery (ribosomes, t-RNAs, amino acids, etc.), and so no matter what the genome structure of the virus, messenger RNA (m-RNA) must be generated that can translate on the host ribosomes.
(i) Double-stranded (ds) DNA viruses:
Animal viruses:
Papovavirus (Papillomavirus, Poliomavirus), Adenovirus, Herpesvirus, Poxvirus; plant viruses: Cauliflower mosaic virus, Dahlia mosiac virus, Carnation etched ring virus, Cassava vein mosaic virus, Mirabilis mosaic virus; bacterial viruses: E. coli bacteriophages T1 ,T2 ,T3, T4 , T5, T6, T7, PM2. Lambda (λ) phages; cyanophages: N1 phage.
(ii) Single-stranded (ss) DNA viruses:
Animal viruses:
Parvoviruses; plant viruses: Bean golden mosaic virus, Maize streak virus, Beat curly top virus, Chlorosis striate mosaic virus; bacterial viruses: E. coli bacteriophages φX174, S13, M12, M13.
(iii) Double-stranded (ds) RNA viruses:
Animal viruses:
Reovirus, Orbivirus, Rotavirus. Blue tongue virus; plant viruses: Wound tumour virus, Rice dwarf virus, Maize rough dwarf virus, Pangola stunt virus, Fiji disease virus: bacterial viruses: Bacteriophage φ6.
(iv) Single-stranded (ss) RNA viruses:
Animal viruses:
Picornavirus (Polio virus), Togavirus, Retroviruses (Sarcoma virus, Paramyxovirus, Orthromyxovirus (Influenza virus). Rhabdovirus; Plant viruses: Tobacco mosaic virus (TMV), Satellite necrosis virus, Lettuce necrotic yellows virus. Sonchus virus. Wheat striate mosaic virus, Potato yellow dwarf virus, and many other plant viruses; bacterial viruses: Bacteriophages MS2, F2, Fr.
(ii) The Envelop:
It is the outermost membranous covering present in some animal viruses (helical and icosahedral both), some plant viruses and bacteriophages, and is usually 100-50 Å thick. ssRNA viruses which possess outer envelope are orthomyxoviruses, rhabdoviruses. coronaviruses, arenaviruses, paramyxoviruses, togaviruses, etc. Bacteriophage φ6 is an example of dsRNA enveloped virus.
The dsRNA enveloped viruses are poxviruses (cytoplasmic assembly), bacteriphage PM2. The envelop of virus resembles with a typical biological membrane and consists of phospholipid bilayer in which proteins are found embedded. It has spikes which are made of glycoproteins.
The spikes may possess haemagglutinating and/or neuraminidase activities. The envelop is a flexible membranous structure and, therefore, enveloped viruses frequently have a somewhat variable shape hence called pleomorphic. Animal virus envelops generally arise from nuclear or plasma membranes of hosts.
The biochemical constituents of an envelope are protein, enzymes, carbohydrates, and lipids.
(i) Proteins:
Protein component of the envelop is of viral origin as well as of the host cell. In few viruses like rhabdoviruses, myxoviruses and arboviruses the envelope-proteins are coded by viral genomes.
No concrete evidences are available about the source of envelope protein in herpesviruses, leukoviruses and poxviruses. Carbohydrate-free polypeptide is present in the envelope of orthomyxoviruses and paramyxoviruses. Presence of glycoproteins have been observed in almost all the classes of enveloped viruses.
(ii) Enzymes:
Animal virus possess different kinds of enzymes and their activities can be observed in enveloped viruses. The synthesis of mRNA in dsRNA viruses is controlled by viral enzymes. Such kind of activity has not been observed in bacterial and plant viruses.
Viral enzymes are responsible for different activities in viruses. Some examples of viral enzymes are RNAase and reverse transcriptase in retroviruses, protein kinase in herps and adenoviruses, DNA dependent RNA polymerase in poxvirus.
(iii) Carbohydrates:
A sufficient amount of carbohydrate specified by either host cell (e.g., arbovirus) or viral genome (e.g., vaccinia virus) is found in viral envelope. Influenza virus, Parainfluenza virus SV5 and Sindbis virus contain galactose, mannose, glucose, fructose, glucosamine and galactosamine in their envelope as carbohydrate. Glycoproteins and glycolipids are the major carbohydrate constituents of enveloped viruses. Carbohydrates, however, are normal host constituents.
(iv) Lipids:
Lipids, like carbohydrates, come from host cells. They provide flexibility to envelop.
The lipids present in viral envelope fall under the four classes:
(a) Phospholipids (e.g., sphingomyelin, phosphatidyl choline, phophatidyl ethanolamine, phosphatidyl serine and phosphatidyl inositol),
(b) Cholesterol,
(c) Fatty acid, and
(d) Glycolipids (e.g., glycosphingolipids made up of sphingosine, fatty acid and carbohydrate).
(iii) Nucleocapsid Proteins:
The viral capsids are made up totally of proteins of identical subunits which enclose nucleic acid and help protecting from nucleases. The helical capsids contain single type of protein and icosahedral capsid contains several types of protein. For convenience, TMV contains single protein types, adenovirus contain 14 protein types. T4 bacteriophage contains 30 protein types, etc.
(iv) Core or Internal Proteins:
Proteins associated with viral nucleic acid are called core proteins. Adenoviruses, Vesicular stomatitis virus and influenza virus contain V and VII type of protein as protein component of nucleic acid.
Essay # 8. Assay of Viruses:
Reasons:
Assay of viruses, the accurate determination of concentration, is a basic and primary pre-requisite for many reasons which are the followings:
1. A virologist must know whether a sample of extract contains a virus or not and how much of it is present in the extract so that he knows whether it is worthwhile to undertake its purification.
2 Assay of viruses helps to know as to which layer during density gradient centrifugation contains the virus, of whether a virus multiplies in an insect vector, or to find out the rate of multiplication and its time course in a host.
3. Accurate determination of virus concentrations help study virus structure, multiplication, and other aspects of their biology.
Methods of Assay:
The concentration of viruses in a given sample can be determined either by counting particle numbers directly (local lesion method, electron microscopic counting) or indirectly (hemagylutination assay) or by measurement of the infections unit concentration or infectivity measurements (plaque assay).
(i) Local Lesion Method:
Crude or purified virus preparation is diluted in water or saline solution in geometrical progression in two-fold (1/2, 1/4, 1/8, 1/16, …, 1/512, etc.) or ten-fold dilution series (1/10, 1/100, …, 1/10,000, etc.) and mechanically inoculated, in the presence of an abrasive or chemical, in a leaf of a test plant. The number of lesions produced by a particular dilution is a major of the amount of virus present.
For the purpose, however finely divided carborundum powder of 400 or 500 mesh is thinly dusted on the upper surface of a leaf and the inoculum is rubbed over the leaf surface followed immediately by washing the leaf with water. Application of in oculums in most commonly done by rubbing with a poster brush, finger or spatula.
Local lesions generally appear in about a couple of days. In some cases they may appear after 18 hours or less and in exceptional cases after 6 to 7 days. Time of counting the local lesions varies greatly and depends upon the virus and the test plant in question.
For convenience, counting of lesions produced by Tobacco mosaic virus (TMV) or been or Nicotiana glutinosa and by cucumber mosaic virus (CMV) on cowpea is normally done after 4 and 2 days of inoculation respectively and gives correct data.
Amount of tobacco mosaic virus (TMV) in inoculum corresponds to the number of lesions produced on the test plant, N. glutinosa, leaf (Fig. 11.5).
This is also essentially true of a large number of other viruses on various test plants. Fig. 11.5 shows the type of curve obtained when virus concentration is plotted against the number of lesions produced. The curve is almost linear in the region of number of lesions ranging from 10 to 100 or 150 and gives an accurate estimate of the virus concentration.
Local lesion method is most successful with sap- transmissible plant viruses like the mosaic and ring spot viruses. Local lesion assays for some persistent viruses like lettuce necrotic yellows virus, pea enation mosaic virus, potato yellow dwarf virus, etc. are also available.
(ii) Electron Microscopic Counting:
Viruses can be counted directly with the electron microscope. There are various procedures employed for the purpose. In one procedure the virus sample is mixed with a known concentration of small latex beads, and sprayed on a coated specimen grid. The beads and virus particles are counted; the virus concentration is calculated from these counts and from the bead concentration (Fig. 11.6).
Electron microscopic counting method often works well with concentrated preparations of viruses of known morphology. Viruses can be concentrated by centrifugation before counting if the virus preparation is too dilute. However, if the latex beads and viruses are not evenly distributed, the final count may not be accurate.
(iii) Hemagglutination Assay:
Hemagglutination assay is the most popular indirect method of counting animal viruses. Many viruses can bind to the surface of red blood cells (RBCs) (Fig. 11.7). If the ratio of viruses to RBCs is large enough, the viruses join the red blood cells together, forming a network that settles out of suspension or agglutinates. In practice, RBCs are mixed with a series of virus preparation dilutions and each mixture is examined.
The hemagglutination titer is the highest dilution of virus (or the reciprocal of the dilution) that still causes hemagglutination. Hemagglutination assay is an accurate, rapid method for determining the relative quantity of viruses such as the influenza virus.
If the actual number of viruses required to cause hemagglutination is determined by another technique, he assay can be used to a certain the number of viruses present in a sample.
(iv) Plaque Assay:
When a virus initiates an infection on a layer of lawn of bacteria, or animal host cells growing on a flat surface, a zone of lysis or a zone of growth inhibition may occur that results in a clear area in the layer or lawn of growing hosts cells.
This clearing is called a plaque, and it is assumed that each plaque has originated from replication events that initiated with one virus. Plaques are essentially “windows” in the lawn of confluent cell growth.
In the plague assay several dilutions of bacterial or animal viruses are plated out with appropriate host cells (Fig: 11.8). When the number of viruses plated out are much fewer than the number of host cells for infect on and when the viruses are distributed evenly, each plaque in a layer of bacterial or animal cells is assumed to have arisen from the multiplication of a single virus particle.
Therefore, a count of the plaques produced a. a particular dilution gives the number of infectious viruses of plaque-forming units (PFU), and the concentration of infections units in the original sample can be easily calculated. For example, suppose that 0 10 ml of a 10-6 dilution of the virus preparation yields 65 plaques, the original concentration of plaque-forming units (number of infectious viruses).
PFU/ml = (65 PFU/0.10 ml) (106) = 6.5. x 108.
Essay # 9. Biological Status (Nature) of Viruses:
The status of viruses as biological agents/organisms remains obscure as has been depicted in the five kingdom system of classification of living organisms by Whittaker. One is equally sure about their being organism or even living being of any kind as about their being non-living.
An intense controversy exist between biologist who consider viruses is organisms and biochemists who consider them as molecules. Nonetheless, viruses possess the properties some of which are traditionally associated with organisms, while others characterize non-living molecules.
I. Animate (Living) Characteristics of Viruses (Organism Concept):
Biologists have regarded organisms on the following four important counts:
(i) Viruses multiply within the host cells and give to the same genetic types, i.e., they reproduce,
(ii)They undergo mutations like living organisms,
(iii) Viruses show extremely specific intracellular parasitism, a characteristic of the living organisms and can be easily transmitted from host to host, and
(iv) Viruses can be easily reconstituted with the help of their already separated components; the reconstituted virus may undergo enzymatic changes in vitro.
II. Inanimate (Non-Living) Characteristics of Viruses (Molecular Concept):
Viruses are not “cells” because they have no cytoplasm, nucleus, membranes, ribosomes, enzymes etc. necessarily present in a cell. They lack the ability to grow independently, and completely depend upon other living organisms for their very existence. Viruses do not have an energy production system of their own.
They can easily be crystallized, precipitated and remain as inert chemicals outside the host cell. These non-living characteristics of viruses enable one to hesitate in accepting them as organisms. The biochemists proclaim that the uniform size, shape chemical composition and crystalizability are the properties of viruses that they share with molecules.
They give following arguments in support of the molecular concept:
(i) Size of the some viruses falls within the range of sizes of protein and nucleic acid molecules,
(ii) Building up of the viral protein monomers and of the protein coats of viruses from protein monomers follow the same general principles of chemistry as in the synthesis of protein molecules,
(iii) Chemical and physical homogeneity of virus particles compares with the homogeneity of protein molecules, and
(iv) Crystallization is a physical phenomenon based on the formation of configurations of minimum energy level. Viruses do seem and behave molecules during their crystallization.
What the viruses are then? What is their biological status? The answer to these questions, as stated above remains controversial and no consensus has yet resulted.
Since the viruses are very important and play’ significant role in the biological system, A. Lwoff’s contention that “THE VIRUSES ARE VIRUSES” appears quite significant when one considers the characteristics of viruses to assign them a proper place in the system of classification of living beings.
Essay # 10. Cultivation of Viruses:
As the viruses do not reproduce independent of living host cells, they cannot be cultured in the same as bacteria and eukaryotic microorganisms.
However, the cultivation of viruses can be discussed under following heads:
(i) Cultivation of animal viruses,
(ii) Cultivation of plant viruses, and
(iii) Cultivation of bacteriophages.
(i) Cultivation of Animal Viruses:
1. In Animal Cells:
Suitable living mammals (such as sheep or calves or rabbits) are selected for cultivation of viruses. The selected animals should be healthy and free from any communicable diseases. The specific virus is introduced into the healthy animals.
The site of administration varies according to the type of virus. The virus is allowed to grow in the living animal. At the end of incubation period, the animals are slaughtered and washed thoroughly and viruses are obtained from them.
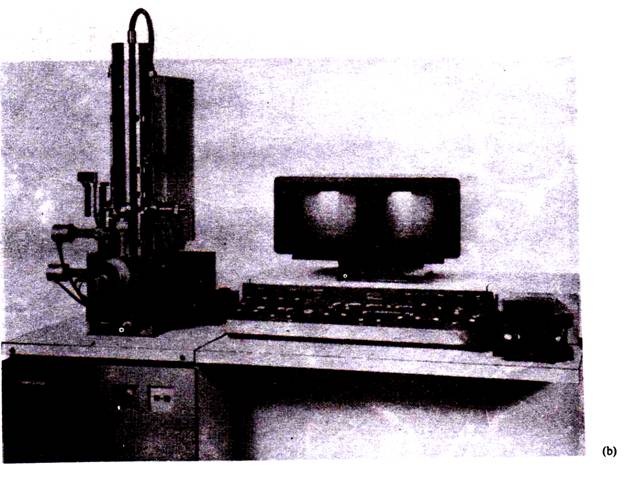
2. In Chick-Embryo:
The animal viruses can be successfully cultivated using chick-embryo technique (Fig. 11.8). In this method fertile hen eggs are selected. Eggs must not be more than 12 days old. To prepared the egg for virus cultivation, the shell surface is first disinfected with iodine and penetrated with a small sterile drill. After inoculation, the drill hole is sealed with gelatin and the egg is then incubated.
Viruses may be able to reproduce only in certain parts of the embryo; therefore, they must be injected into the proper region. For convenience, the inayxoma virus grows well on the chorioallantoic membrane, whereas the mumps virus prefers the allantoic cavity. The infection may produce a local tissue lesion known as pock, whose appearance often is characteristic of the virus.
3. In Vitro Culture (Tissue Culture Technique):
More recently developed in vitro cultivation of animal viruses has eliminated the need to kill the animals. This technique has become possible by the development of growth media for animal cells and by the availability of antibiotics which prevent bacterial and fungal contaminations in cultures.
Cultivating animal viruses using tissue culture technique involves following three main steps:
(i) Monolayer Preparation.
Live tissues of vital organs (e.g., heart or kidney) are taken and the cells are separated from the tissue by digesting the intracellular cement substance with dispersing agents such as trypsin’s or collagenase or ethylenediaminetetraacetic acid (EDTA). The cell suspension is passed through screen filters so that the coarse particles are removed from the separated cells. The cells are washed free of dispersing agents.
The cells are centrifuged if required and re-suspended in nutrient medium contained in glass or plastic vessels. The composition of medium and other conditions of incubation depends on the type of cells used. Upon incubation the cells quickly settle and attach firmly to the bottom of the flask. If undisturbed, these cells grow and spread to form monolayers.
(ii) Clonal Cell Line Preparation:
The monolayer cells are first removed and washed with saline solution devoid of calcium and magnesium ions and then added to the dilute solution of EDTA (1: 3000) to chelate intracellular magnesium or calcium ions. After sometime, the loosened cells are shaken and re-suspended in growth medium in fresh culture vessels and incubated. The cells are cultivated under 5% CO2 condition.
The cultures of cell obtained so are called diploid cell strain. It is extremely difficult to distinguish primary cell and the diploid cell strain. On repeated sub culturing, each cell starts multiplying to form separate colony. If each colony is removed and cultivated separately, it forms pure culture. This bunch of cells from single cell is called clonal cell lines.
(iii) Infection with Virus:
The clonal cell lines suspended in suitable media are infected with any desired virus which replicates inside the multiplying cells. If the virus is virulent, they cause lysis of cells and virus particles are released in the surrounding medium. These newly produced virus particles (virions) infect the adjacent cells.
As a result localized areas of cellular destruction and lysis (called plaques) often are formed. Plaques may be detected if stained with dyes, such as neutral red or trypan blue that can distinguish living from dead cells. Viral growth does not always result in the lysis of cells to form a plaque.
Animal viruses, in particular, can cause microscopic or macroscopic degenerative changes or abnormalities in host cells and tissues which is called cytopathic effects; cytopathic effects may be lethal, but plaque formation from cell lysis does not always occur.
(ii) Cultivation of Plant Viruses:
There are several methods of cultivation of viruses such as plant tissue cultures, cultures of separated cells, or cultures of protoplasts, etc. Viruses also can be grown in whole plants.
Leaves are mechanically inoculated by rubbing with a mixture of viruses and an abrasive such as carborundum. When the cell wall are broken by the abrasive, the viruses directly contact the plasma membrane and infect the exposed host cells. (The role of the abrasive is frequently filled by insects that suck of crush plant leaves and thus transmit viruses.)
A localized necrotic lesion often develops due to the rapid death of cells in the infected area. Even when lesions do not arise, the infected plant may show symptoms such as change in pigmentation or leaf shape. Some plant viruses can be transmitted only if a diseased part is grafted onto a healthy plant.
(iii) Cultivation of Bacteriophages:
Bacterial viruses of bacteriophages (phages for short) are cultivated in either broth or agar cultures of young, actively growing bacterial cells. Several host cells are destroyed that turbid bacterial cultures may clear rapidly because of cell lysis.
Cultures are prepared by mixing the bacteriophage sample with cool, liquid agar and a suitable bacterial culture medium. The mixture is quickly poured into a petri dish containing a bottom layer of sterile agar.
After hardening, bacteria in the layer of top agar grow and reproduce, forming a continuous, opaque layer or lawn. Wherever a virion comes to rest in the top agar, the virus infects an adjacent cell and reproduces. Finally, bacterial lysis generates a plaque or clearing in the lawn.
Essay # 11. Transmission of Viruses:
The modes of spread or transmission of plant viruses from host to host are of various types and take place by:
(1) Mechanical means,
(2) Vegetative propagation,
(3) Grafting,
(4) Seeds,
(5) Fungi,
(6) Dodder,
(7) Pollen,
(8) Soil,
(9) Nematodes, and
(10) Insects.
(i) Transmission by Mechanical Means:
Mechanical transmission of viruses can be considered as of two types:
(i) Mechanical transmission in fields:
In fields, the transmission of viruses takes place between growing plants. The strong wind may bring rubbing of leaves together, the latter may exchange their saps through wounds.
The viruses present in the sap are transmitted from infected to the healthy one. This type of transmission is most common in plants infected by potato virus-X. Besides, contaminated hands, cloths, pruning shears and other instruments also cause transmission of viruses.
(ii) Mechanical transmission in laboratories:
This is actually the inoculation of viruses in laboratory. Cells of the infected plant parts are broken generally with the help of mortar and pestle resulting in the release of cell– sap containing virus particles.
If desired, this sap is centrifuged to remove fragments of host cells. The sap is applied to the wounded leaf surface with the help of gentle rubbing Viruses enters the host through wounds and starts new infections on healthy plants.
(ii) Transmission by Vegetative Propagation:
Vegetative propagating organs of infected plants e.g., cutting, tubers, bulbs, corms, etc. already possess viruses in them and when grown transmit them to the new generation.
(iii) Transmission by Grafting:
Grafting plays an important role in virus transmission. Generally the ornamental plants and fruit trees are propagated by grafting and the viruses are transmitted from infected plant to the healthy one through continuous channel of xylem, phloem and plasmodesmata formed between the two grafted plants for water, nutrients and cytoplasmic movements.
(iv) Transmission by Seeds:
It is considered that about 100 viruses are transmitted by seeds. Bennett (1969) prepared a list of 63 viruses from 124 species transmitted by seeds. Viruses may be present on external surfaces of the seeds, may be present internally in testa, endosperm or embryo of infected seeds. Ex-Bean common mosaic virus.
(v) Transmission by Fungi:
It is limited to few fungal genera; only 5 genera of fungi, e.g., Olpidium Synchytrium. Polymixa, Spongospora and Pythium are reported to transmit the viruses to healthy plants. These fungi generally acquire the virus particles from inside the host cells and retain them on or within the body of their zoospores and resting spores. While causing infection on new host plant the spores transmit the viruses.
For convenience, following are the names of fungal species and some viruses transmitted by them:
(i) Olpidium brassicae: Tobacco nacrosis virus, Tobacco stunt virus, Lettuce big vein virus and Satellite tobacco necrosis virus.
(ii) Olpidium cucurbitacearum: Cucumber necrosis virus.
(iii) Synchytrium endobioticum: Potato virus.
(iv) Polymixa graminis: Wheat mosaic virus.
(v) Spongospora subterranea: Potato mop top virus.
(vi) Pythium sp.: Beet necrotic yellow vein virus and pea false leaf-roll virus.
(vi) Transmission by Dodder:
A parasitic plant called dodder (Cascuta sp.) plays an important role in transmission of several plant viruses, because this parasitic plant forms a bridge between the two plants. The viruses move from the vascular bundle of infected plants to the food stream of Cuscuta through the haustoria of the latter. They are translocate through the phloem and are transmitted to the next plant through newly developed haustoria.
The movement of viruses from vascular bundle to the haustoria of Cuscuta is understandable but the mechanism of movement of viruses from haustoria to the vascular bundles of newly infected plant needs clear explanation because the movement of viruses is against the prevailing direction of food movement.
(vii) Transmission by Pollen:
The transmission of virus by pollen is quite rare. Pollen-carried viruses infect the floral parts of healthy plant and the whole plant becomes infected gradually due to the systemic movement of viruses.
(viii) Transmission by Soil:
Mayer (1886) advocated the soil transmission of tobacco mosaic virus on the ground that this virus is quite stable and may retain its infectivity for a long time in the infected leaf fragments which fall and remain in the soil. Recently, addition of some chemicals in the soil check the spread of viral diseases has given some encouraging results.
(ix) Transmission by Nematodes:
Nematodes receive the viruses from infected plant roots while feeding on them and they transmit the viruses to the roots of healthy plants at the time of feeding; about 12 plant viruses are in the knowledge which gets transmitted by one or more species of genera Langidorus, Xiphinema and Trichodorus.
Some viruses transmitted by these ectoparasitic, soil inhabiting nematodes are viz., tobacco ring- spot virus, tomato black-ring virus, cherry leaf-roll virus, tobacco rattle virus etc.
(x) Transmission by Insects:
It is the most common method of virus transmission in the field. Aphids and leafhoppers are considered to be the most important vectors because they account more than 80% of all insect vectors.
About 300 aphid species transmit about 200 viruses from plant. Similarly, about 130 leafhopper species transmit about 75 viruses. Besides aphids and leafhoppers, white flies, scale insects, treehoppers, battles, grasshoppers etc. also transmit plant viruses.