Transduction is of two types, generalized transduction and specialized transduction.
Type # 1. Generalized Transduction:
Generalized transduction was discovered in 1952 by Norton Zinder and Joshua Lederberg. They were repeating the experiments of Lederberg and Tatum (1946) on conjugation that occurred in E.coli K12 taking another bacterium Salmonella typhimurium.
They selected the following two strains of S. typhimurium:
(a) The LA22 Strains:
This strain was unable to synthesize the amino acids, phenylalanine and tryptophan (Phen– Trp– strain) but could synthesize methionine and histidine (Met+ His+ strain).
(b) The LA2 strain:
It was unable to synthesize methonine and histidine (Met– His– strain) but could synthesize phenylalanine and tryptophan. It is written as LA2 Met– His– Phen+ Trp+. They found that a mixture of two autotrophic strains resulted in prototrophs at the rate of 1/ 105 cells.
The wild type prototrophs could synthesize all the four amino acids (Phe+ Trp+ Met+ His+). Though genetic recombination occurred in S. typhimurium, yet it was not as a result of conjugation which was confirmed later on.
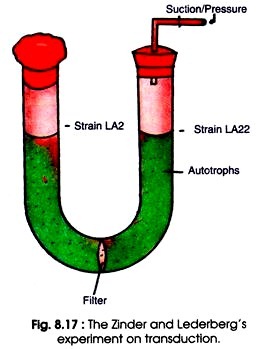
Each strain was added in an arm of U tube. The two arms of U-tube were separated by a bacteria proof sintered glass filter which allowed free movement of nutrient media but not bacteria (Fig. 8.17). After applying alternate sanction and pressure the culture medium was allowed to pass from one arm to the other.
Thereafter, the two auxotrophs present in two separate arms were grown in the same culture medium. The prototrophs were recovered from LA22 culture but not from LA2 culture. From LA2 strain a genetically active filterable agent was produced which formed prototrophs in LA22. It was confirmed that the filterable agent was larger than DNA and resistant to DNase.
Later on the filterable agent was confirmed as temperate Salmonella phage P22. The P22 was carried in one of the parental strain as prophage. Its presence was ascertained by the fact that it could be destroyed by treating with P22 antiserum.
Generally, P22 exists in lysogenic state in LA22 strain of S. typhimurium. Generalized transduction occurs during the lytic cycle of virulent or temperate phages. Outline of generalized transduction is given in Fig. 8.18.
After penetration (A) phage genome multiplies within the cell (B, C), the bacterial chromosome is fragmented into pieces (C). During assembly when virus DNA is packed into protein capsid, by mistake the random fragment of partially degraded bacterial chromosome is also packed (D).
Since the upper limit of DNA to be packed in capsid is about 44 kb, some or all viral DNA is left behind. The quantity of bacterial DNA carried by phage DNA depends mainly on the size of capsid. Phage P22 carries about 1% of bacterial chromosome and PI phage of E. coli can carry about 2-25% of bacterial genome. The frequency of such defective phage is about 10-5 to 10-7 of the total progeny phage produced. This defective phage is also called generalized transducing particle.
When this defective phage infects another bacterium, the genome is introduced within the host cell (Fig. 8.18 E). The transferred bacterial DNA (exogenote) is integrated into the recipient bacterial chromosome (endogenote) (F). In addition, 70-90% of transferred DNA is not integrated with the recipient chromosome but survives and expresses itself (G).
Generalized transduction also helps the mapping of bacterial genes because the chromosomal segment which has been transferred by bacteriophage contains hundreds of genes. These are tested by using the genetic markers. For example, two temperate (PI and 363) transfer markers from one strain of E. coli to the other.
When coliphage P1 is grown in a wild type E. coli cells that utilizes threonine, leucine and sodium azide (Thr+ Leu+ Azi+) and then used to infect a Thr– Leu– Azi– recipient auxotroph, only 3% of threonine prototrophs (Thr+ Leu– Azi–) were produced.
A few recombinants were leucine prototrophs (Thr+ Leu+ Azi–). This shows that only a short segment of bacterial DNA is involved and usually only a single marker locus is produced. Rarely, multiple transductions (transduction of several gene loci) occur. This also shows that Thr+ is closely linked to leu+ but not azi+. Therefore the order of linkage is thr+ leu+ azi+.
Abortive transduction:
When a segment of bacterial DNA (exogenote) is introduced into another bacterium by a bacteriophage, the exogenote may be integrated into the recipient bacterial chromosome (Fig. 8.18 E-F). This type of transduction is called complete transduction.
In contrast, when the exogenote is not integrated into the endogenote and remains free, it is called abortive transduction (H). The recipient bacteria that contain this non-integrated transduced DNA and are partially diploid, are called abortive transductants. When the abortive transductant divides, out of two, only two daughter cells contain exogenote in each generation. The other cells do not contain the exogenote.
The cells containing exogenote synthesize the functional enzymes because the transduced DNA specifies the normal complement by enzymes. However, in non-transduced cells the synthesis of functional enzyme gradually decreases with the progress of cell multiplication. This results in formation of slow growing small colonies or micro colonies.
Type # 2. Specialized (Restricted) Transduction:
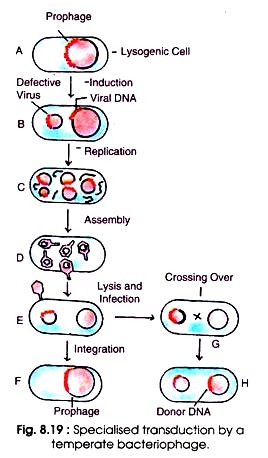
Certain temperate phages can also transfer only a few restricted genes of the bacterial chromosome to the recipient bacterial cell. This transfer of bacterial genes adjacent to prophage only to the recipient chromosome is called restricted or specialized transduction (Fig. 8.19 A-H). For the first time Morse (1956) discovered specialized transduction. It is made possible by an error in the lysogenic life cycle of phage.
When a phage genome is introduced in the bacterial cell, it becomes integrated with bacterial chromosome as prophage (A). Upon induction the DNA becomes free containing a small segment (about 5 to 10%) of bacterial chromosome (B). It multiplies and disintegrates the bacterial chromosome (C). After assembly of phage DNA plus bacterial chromosome, the bacteriophage is released from the bacterial host (D).
Usually this phage is defective and lacks some part of its attachment site. When this defective phage infects a bacterium it introduces its DNA containing a piece of bacterial chromosome (E). The defective phage cannot reproduce without the assistance of helper phage.
The genes of the phage can insert with homologous DNA of the infected bacterium (F). Sometimes crossing over occurs between the homologous gene loci of the bacterial chromosome and the donor DNA attached with phage genome (G) resulting in integration of the donor DNA with the recipient DNA (H).
(a) Low frequency transducing (LFT) lysates:
The mechanism of specialized transduction and production of LFT are shown in Fig. 8.20. The best studied example of specialized transduction is the phage λ of E. coli. The genome of phage λ is inserted into the bacterial chromosome at attachment (att) site. The att sites of both the phage and bacterium are similar but not identical.
They can complex with each other. The insertion of phage genome in the bacterial chromosome takes place always between the genes of E. coli e.g. gal (galactose locus) and bio (biotin locus). When prophage is excised out from E. coli chromosome, it sometimes takes out with it gal or bio genes.
This occurs due to improper excision of integrated prophage DNA as it occurs in the formation of F factor. Thus after cell lysis resulting from induction of lysogenized E. coli, some normal phages and a few defective transducing particles are released.
These particles are called λdgal (λ defective gal) because they contain galactose utilizing gene. Since the number of defective particles in cell lysate (product) is low (10-5 to 10-6), it is called the low frequency transduction (LFT) lysates.
The defecting transducing particles possess a non-functional hybrid integration site which is in some part bacterial in origin and some part phage in origin. They lack some portion of phage genome, but they are capable of transducing and integrating into a new host carrying the original host genes with it. This establishes a merodiploid condition.
(b) High frequency transduction (HFT) lysates:
If there is a normal phage λ genome in the same cell, the defective phage λ carrying the gal gene can integrate. The normal phage integrates resulting in two bacterial phage hybrid att sites where the defective λdgal can insert phage that acts as helper phage because it helps the integration and reproduction of the defective phage (Fig. 8.21). The transductants are unstable because the prophage can be excised by certain stress like UV radiation.
The UV radiation results in new phages if a normal phage λ has also lysogenized this cell. Hence, induction of this double lysogen (dislysogen) produces high frequency transduction (HFT) lysates containing about 50% λdgal and 50% phage λ. In constrast to LFT lysate, it is shown as HFT lysate because the number of HFT particles is very effective in transduction.
The HFT lysates contain transducing particles with a frequency of about 0.1 to 0.5%. However, phage λdgal infects the recipient cells containing gal bacterial chromosome (gal recipients). Crossing over occurs at the homologous gal sites than heterologous att. sites. This results in production of stable transductants (B).