In this article we will discuss about the co-linearity between genes and proteins.
The most convincing demonstration of the relationship between genes and their product proteins came from two separate investigators, one by C. Yanofsky and his colleagues working on the tryptophan synthetase system in E. coli, the other from Sarabhai and his team who were studying bacteriophage.
Yanofsky (1967) showed the existence of a co-linear relationship between a gene and its protein structure. The tryptophan mutant in E. coli (trp–) cannot grow on minimal medium until the amino acid is supplied from outside.
In fact it was found that the trp– mutants could be defective at one of a number of places in the trp biosynthetic pathway and the mutants were likewise named trp A, trp B, trp C and so on. The enzyme tryptophan synthetase is composed of two chains A and B, each produced by a different gene, that is gene A and gene B respectively.
Yanofsky et al. first focused on gene A and mapped the sites of several mutations within gene A which specified the sequence of the polypeptide chains. They also determined the positions of the changed amino acid within the polypeptide chain of each of the mutant strains. Their earlier work had already established that the mutant polypeptide differed from the wild type protein by a change of a single amino acid.
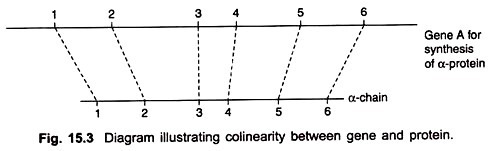
It was clear now that the sequence of the changed amino acids within the polypeptide chain was the same as the sequence of mutations that gave rise to the changed amino acids (Fig. 15.3).
The genetic distances between the sites of mutations were proportional to the distance along the polypeptide chain between the altered amino acids. In other words, the positions of the mutant sites in gene A and of changed amino acids in the protein product were co-linear.
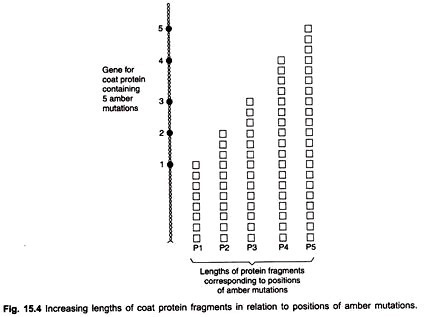
Sarabhai et al. (1964) analysed co-linearity using amber mutants in the bacteriophage T4. In the class of mutants known as amber, translation of the genetic message is terminated at the site of mutation, so that only fragments of polypeptides are produced.
When amber mutations occur in the genes specifying coat proteins, the new points of chain termination cause only N-terminal portions of the chain to be made. If amber mutants are grown on certain special host bacteria designated as permissive, translation is not interrupted by chain termination and near normal phenotypes are produced.
Sarabhai (1964) found that the lengths of the head polypeptide fragments produced by the various head amber mutations could be correlated with the genetic location of the mutation. Mutations near one end of the head gene produced short fragments. When the site of mutation was located further away from this end, the fragment length increased, thus confirming the concept of co-linearity (Fig. 15.4).