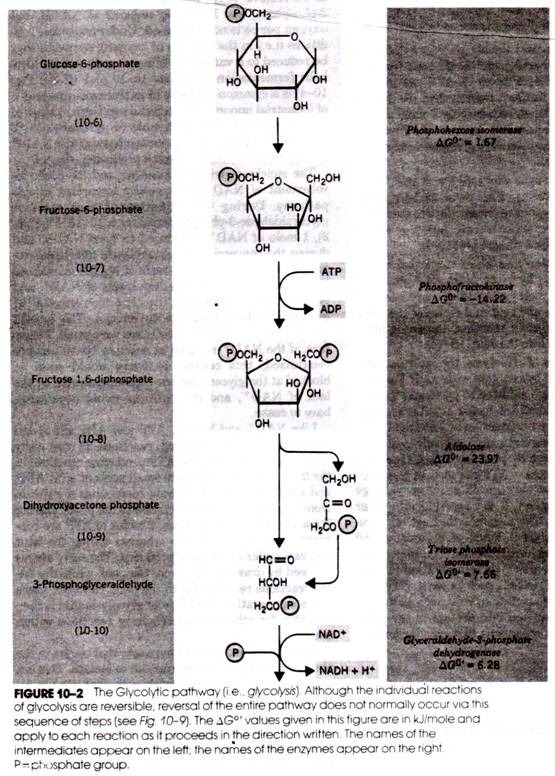
The reactions of glycolysis have no specific requirement for oxygen. Oxidation reactions do occur, such as the removal of two hydrogen’s from glyceraldehyde- 3-phosphate, and NAD+ is reduced to NADH, but oxygen per se is not consumed.
Under anaerobic conditions (i.e., in the absence of oxygen), pyruvate may be reduced to a variety of different compounds.
Alcoholic fermentation (reactions 10-19 and 10-20, Fig. 10-4) is a common pathway in microorganisms and is of industrial importance.
In these two steps, 1 mole of CO2 is removed from each mole of pyruvate (i.e., 2 miles of CO2 per mole of monosaccharide) and NADH is reoxidized to NAD +, thereby producing ethanol.
The stoichiometric relationships and the cyclic involvement of NAD are especially important in this pathway. During the oxidation of each mole of glyceraldehyde-3-phosphate (reaction 10-10, Fig. 10- 2), 1 mole of NAD+ is reduced to form NADH; and during the conversion of acetaldehyde to ethanol (reaction 10-20, Fig. 10-4), a mole of NADH is oxidized to form NAD+.
Therefore, the levels of NAD+ and NADH are unaffected by the conversion of glyceraldehyde-3-phosphate to ethanol. The quantities of these coenzymes in cells are very small. Therefore, if the NAD + reduced in reaction 10-10 was not reoxidized, this central pathway would soon be blocked at the glyceraldehyde-3-phosphate step by the lack of NAD+ and the pathway would necessarily have to cease.
Like NAD+ and NADH, ATP and ADP are cycled between the ATP-requiring reactions in the early steps of glycolysis and the ATP-producing reactions in the later steps. Cells contain small pools of ATP, ADP, and AMP; when needed, these compounds are drawn from the pools and later returned to the pools.
During the early stages of glycolysis, 2 moles of ATP are consumed per mole of glucose, whereas during the later stages 4 moles of ATP are produced. The early stages proceed by drawing ATP from the pool, while the latter reactions return ATP to the pool. However, there is a new generation of ATP from glycolysis that is then available for other energy-requiring reactions within the cell.
Another common fate of pyruvate that occurs in the absence of oxygen is its conversion to lactate. This is a normal process in active muscle cells that are not receiving adequate amounts of oxygen and in many plant and bacterial cells that live under anaerobic conditions.
In a single reaction, pyruvate is converted to lactate (reaction 10-21, Fig. 10-4). During the reaction, 1 mole of NADH is converted to NAD+. This stoichiometrically balances NADH production during the earlier glyceraldehyde oxidation step.