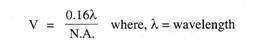1. General:
Growth related intracellular events as shown in Fig. 2.4 are the following:
(a) Anabolic aberration and spontaneous mutations.
(b) Biosynthetic error.
(c) Post biosynthetic damage in cells.
During growth of cells intracellular proteins in wild microbial strains are broken down to appropriate rates during starvation or endogenous metabolism. Even in exponential phase of growth of bio cells in vivo proteolysis has been shown to be possible (Fig. 2.4).
It necessitated proper understanding and study of the following:
i. Molecular principles of protein design/biosynthesis vis-a-vis proteolysis in cells.
ii. In vivo proteolysis kinetics.
iii. Influential parameters of in vivo proteolysis.
iv. Foreign protein expression and/or secretion in cells.
In microbial cells for obtaining maximum metabolic economy it is essential to minimize and control in vivo proteolysis. Among several reasons of aging of cells and reduction in productivity, in vivo proteolysis is an important concern. The functional proteins generated through in vivo synthesis in cells are the major determinant in reactions, transport and physical and morphological characteristics of the cell.
Generally the intracellular proteins in wild strains of microorganisms are broken down at appreciable rates during starvation or endogenous metabolism. Thus in vivo proteolysis in cells occurs to prevent the accumulation of highly abnormal proteins that may result from spontaneous mutations, biosynthetic errors or post synthetic damage in cells.
This has direct impact on the bioprocessing objective particularly when the overall objective in a number of contemporary manufacturing biotechnologies and many of those expected in the future is maximal production of a particular protein. In studies with different microorganisms including E. coli for proteolysis it appeared that protein label may appear metabolically stable in growing cells sometimes after the label was provided.
It necessitated proper estimation of intracellular proteolysis and turnover. The method of steady-state measurement of the turnover of amino acid in the cellular protein of growing cells of E. coli has shown the existence of biphasic kinetic regimes in intracellular proteolysis.
It has been observed that when exponentially growing bacteria are deprived of nitrogen, a carbon source or a required amino acid the rate of catabolism of cellular protein increases several fold thereby pointing whether intracellular proteolysis need be considered in relation to protein synthesis. Protein synthesis in overall terms has been considered in William’s structured growth model.
Genetic regulation of synthesis of a particular protein has been studied by several biochemical engineering laboratories. In bacteria as in animal cells in vivo proteolysis requires metabolic energy and is an ATP-dependent process. It has been stated that ATP stimulates proteolysis.
Continuous culture experiment of Willets could suggest that intracellular proteolysis occurs not only in non-growing cells but limited proteolysis occurred during exponential phase as well. The mechanism of intracellular proteolysis in microbial cells is not clearly understood.
However, participation of enzyme-like processes in the reaction has been shown by various investigators and it necessitates the participation of ATP. Therefore, it is important to understand and to describe in quantitative reaction engineering terms the kinetic, regulatory mechanisms of in vivo proteolysis so that protein composition of organisms used in industrial processes can be suitably regulated and controlled.
2. Molecular principles of protein synthesis and degradation in cells:
The efforts of specialists in the kinetics for increased production capacities of microorganisms in biotechnological industries has been diverted strongly in the optimization of cell culture conditions for intracellular protein synthesis but without considering its in vivo degradation.
In order to increase the metabolic economy of the cells in vivo proteolysis must be regulated. Much experimental data has been accumulated which characterizes the influence of various physical and chemical parameters on the specific cell growth rate on different biosynthetic processes.
External character of the influence of temperature, pH, eH values and occasionally pO2 values is fairly well known. The optimized conditions of these parameters in a well nourished medium lead to cell growth via intracellular protein synthesis based on molecular level principles.
Although some simplifying principles may be set forth, exceptions stem from the fact that microbes, particularly bacteria because of extensive inherent control/regulatory mechanisms can adapt to synthesize proteins in many environments and meet diverse challenges in various ways.
Simplifying Principles:
The growth of a simple microorganism even as simple as E. coli is dependent on numerous reactions and functions of many individual control or regulatory molecular level mechanisms. Molecular level principles of protein synthesis may be ascribed to the following (Fig. 2.5).
A. Molecular pool, cellular transport and translation:
The microbial regulatory mechanisms function to maintain relatively constant rates of polymerization of macromolecules, e.g., DNA, RNA and protein independent of growth rates. Underlying this are allosteric control mechanisms over metabolic pathways that serve to maintain intracellular pool, active and passive transport mechanisms functioning for the same purpose and regulating the initiation of the synthesis of macromolecules that prevent the inappropriate drain of intermediates.
B. Chain elongation, modulation and molecular catalysis:
In rapidly growing cells the available catalytic units for macromolecular synthesis are nearly maximally utilized. Thus a shift to more rapid growth requires the formation of more ribosomes, and possibly more RNA polymerase. In slowly growing cells, the cells have an excess of ribosomes and polymerase and as yet unknown mechanisms limit their initiation of function. Slowly growing cells can respond to shift up by an immediate increase in the rate of RNA and protein synthesis. The controls of slowly growing E. coli seem to have evolved to provide rapid adaptability whereas those of rapidly growing cells evolved to provide efficiency.
C. Catalytic proportionality and transcription:
In rapidly growing cells, the concentration of ribosomes increases approximately in proportion to the specific growth rate (n). The rate of rRNA and tRNA synthesis appears coordinated. Their rate is the product of the total rate of cellular RNA synthesis and the viable fraction of the synthesis given to the formation of the stable species.
Under certain conditions, turnover of some nascent rRNA has been reported to influence the amount accumulating. This is a feature of normal cell growth as well, at least at low growth rates. Also, tRNA is more stable and increases slightly relative to rRNA at low growth rates.
It has also been seen that the synthesis of rRNA in vivo is generally correlated with the inverse of concentration of guanosine 5′ diphosphate 3′ diphosphate (ppGpp) nucleotide (PG). In vitro the transcription of rRNA from phage templates is initiated by ppGpp at physiological concentrations and this may be the basis for control over the genes of the protein forming system in cells. In addition or as an alternative to active control over the expression of these genes by ppGpp, a passive mechanism has also been envisaged.
The progress that the genes of the protein forming systems are regulated indirectly as a consequence of growth rate depend upon changes in the global pattern of transcription of the genome defined by relative promoter strengths and the effects of repressors and activators operating on the operons.
The synthesis of ribosomal and other proteins of the protein forming system follow a pattern of regulations similar to that of rRNA. Underlying this there must be at least partially distinct mechanisms, since the transcription of the r-protein gene is regulated relative to rRNA transcription.
D. Biokinetic determinant:
The total rate of RNA synthesis is the most crucial parameter of intracellular protein synthesis and hence of growth as it is a determinant of the rate of rRNA synthesis. Shift up of slowly growing cells indicate the presence of reverse transcriptional capacity, whereas that of already rapidly growing cells do not. It is possible that the concentration of RNA polymerase of ATP and GTP, which are needed for initiation and possibly of ppGpp determine the total rate of synthesis.
E. Signaling and control:
It appears that ppGpp plays a significant role in the regulation of enzymatic and transcriptional activities including rRNA formation. The synthesis of ppGpp is related to the differential signal between the rate of provision of aminoacyl tRNA’s and the rate at which they are removed in protein synthesis.
This takes place through a stringent control system. Presence of ppGpp is also affected by a less well-defined metabolic regulation sensitive to the carbon/energy supply. Function of ribosome, may therefore be considered as sensory organelle and restricted to transients of cell growth.
F. Evolutionary/revolutionary continuum:
The growth and protein synthesis in simple bacterium like E. coli may be viewed as continuum of ribosomal synthesis and formation of proteins. Genomic replication and cell division may be quasi-independently regulated processes triggered by events in protein synthesis.
These two processes and other partially autogenous processes through evolutionary/revolutionary continuity and taxation of intermediary metabolism influence the growth rate. The growth of microorganisms with exquisite and complex life cycles may include alternative modes of signaling and control about which little is known and much evolutionary/revolutionary search is awaited.
Although the above principles of in vivo protein synthesis is well known, it is not exactly known whether the same set of principles and parameters of the medium condition and molecular mechanism play a role in vivo proteolysis. It has promoted intensive search in the area.
Catabolic intracellular molecular phenomena in E. coli have been observed to manifest the following:
(a) Bioprocessing of proteins—for cleavage of leader peptides for transported proteins.
(b) Defective protein and peptide manipulation—for degradation of non-functional or mutated proteins or protein containing amino acid analogues in order to increase the metabolic efficiency by recycling required amino acids.
(c) Degradation of functional proteins—for specific metabolic conditions in growing cells. Most native proteins have half lives much longer than the generation time, but a few are remarkably unstable.
Protein catabolic manifestation in E. coli has been shown to depend on the phase of growth rate. Protein catabolism increases several fold in endogenous phase, i.e. on starvation for carbon, nitrogen or amino acids. The above response appeared to be signaled by a lack of complete complement of aminoacyl tRNA.
Thus proteolysis is regulated by principles similar to those regulating rRNA synthesis. The high rate of proteolysis during starvation required continued presence of a high level of ppGpp. In amino acid and/or energy deprivation ppGpp level seems to signal the acceleration of proteolysis.
3. Influential factors:
(i) Nutrient Limitation:
Early Studies of intracellular protein breakdown indicated that proteins were stable to a high extent in growing bacteria although limited proteolysis could be observed in exponentially growing cells of E. coli in a defined medium. The cells subjected to starvation, however, exhibited overall proteolysis at rates of 4 to 1% per hour.
Enhancements of proteolysis were also observed for batch cultures of E. coli entering stationary phase. Organisms like E. coli, Dictyostelium discoideum, Bacillus cerus, B. megaterium, Pseudomonas saccharophila and many others have proteolysis rates within these limits.
Protein turnover in starving yeast is, however, remarkably low and below 1% per hour. It has also been stated that protein synthesized during starvation are much more unstable than the original cell protein. It was seen that 25-35% proteins made under starvation conditions decomposed within 20 minutes.
Pine observed that glucose depletion stimulated intracellular proteolysis and that growth rate influences protein degradation. Cells growing in glycerol limited medium exhibited more rapid breakdown than cells growing more rapidly in medium supplemented with glucose, casein hydrolysate and vitamins.
Thus, Goff and Goldberg noted that acceleration of protein catabolism in poor environments is not correlated with a loss of cell viability nor is it an all-or-none response. In fact, rates of breakdown vary in cells growing at different rates. However, to date there has been very little systematic study of parametric influences on protein degradation kinetics of nutrient levels and limitations and other growth parameters such as temperature.
A thorough literature review revealed very few investigations on proteolysis in chemostat culture, in spite of the advantage for maintaining a steady-state culture over a long term and for investigation of a broad range of growth rates. Willett observed a small, rapidly degraded proteins fraction of E. coli grown in a chemostat.
He found identical overall rates of protein degradation at different dilution rates giving generation times of 1,2 and 4 hours, but noted that sudden decreases in dilution rates gave a transient increase in proteolysis. St. John et al. found that 50% of the proteins in a chemostat culture of E. coli were degraded within about 48 hours at a dilution rate of 0.0478 h-1 (doubling time of 14.5 h).
A double labeling protocol showed that a fraction of the cell protein was five times more labile than another fraction. Soluble proteins were apparently those most susceptible to proteolysis. This small dilution rate likely gave very low residual glucose levels in the chemostat.
It is known that limitation or deprivation of different nutrients can influence intracellular proteolysis in different ways. For example, amino acid starvation elicits a response different from deprivation of an energy source. Phosphate or magnesium limitation has different effects on protein degradation than glucose or amino acid limitation. How these degradation rates are influenced by different types of nutrient limitations, and limitations of different degrees, however, has not been shown.
(ii) Temperature:
Rates of proteolysis have been observed to increase rapidly as the temperature is increased. In E. coli overall intracellular proteolysis at 43-45 °C occurs at a rate similar to that observed under nitrogen starvation. Careful experiment has further shown that temperature changes induce transient fluctuations in the relative rates of synthesis of different proteins which greatly vary by as much as a factor of 50. It is likely to be a consequence of a different influence of temperature on synthesis or degradation or both of these intracellular proteins.
(iii) Oxidants:
Intracellular protein degradation in presence of oxidants has revealed that oxidative damage to proteins may be an important factor. Stadtman and coworkers have proposed that inactivation by oxidants may be a specific mechanism initiating the breakdown of critical cell proteins such as glutamine synthatase in E. coli. This enzyme plays a key role in the flow of ammonia derived nitrbgen into organic compounds and its activity as well as intracellular content is subject to elaborate regulation.
Intracellular breakdown of glutamin synthatase has been suggested to occur in the following two steps:
(1) The enzyme is first inactivated irreversibly by a mixed function oxidase system as occurs in model in vitro studies with ascorbate, iron and oxygen.
(2) The inactivated enzyme is more susceptible to proteolytic attack and is rapidly degraded by some cellular protease.
4. Current needs:
For maximization of metabolic economy of cells regulation/control and optimization of in vivo proteolysis plays important role in developing newer bio-products. Also, in relation to metabolic economy in vivo proteolysis of aberrant proteins is desirable where as that of functional proteins are undesirable. This is reaction, transport and physical, morphological and ontogenetic characteristics of bio-cells in producing bio-cellular products.
It is evident therefore, that engineering biology’s linkage with process biotechnology is inseparable. Close research in these two applied biotechnology areas is ensuing in recent years. In order to understand this intimate linkages between engineering biology and process biotechnology, most advanced techniques of modern age like Artificial Neural Network (ANN), Bio Expert System (BES), Artificial Intelligence (AI) and Advanced computing for life sciences are being used.
The major objectives of developing these techniques are for bioprocessing control, on line diagnosis of bioprocessing systems, computer aided design of biomolecules, recognition of specific bio-molecular sequence, analysis of biochemical switching etc.
Thus, a new wave of technological revolution under the banner of Modern Biotechnology which is hardly twenty years old connotes the use of any living organism or components thereof in the production and processing any of the manufactured products of commercial importance.
In the constitution of linkage of engineering biology and process biotechnology through the use of land marks in modern biotechnology, microbial factories, plant agriculture, animal cell culture bioprocessing, medical engineering sciences and technology, energy and environment provide progress foundations.