The Absorption of Light by Chlorophyll:
The absorption of electromagnetic energy by any atom or molecule often involves a shift of electrons from one atomic orbital to another.
Each electron possesses energy, and the amount is determined by the location of the electron orbital in space and the velocity at which the electron moves.
When an atom absorbs light energy, an electron is either raised to an orbital of higher energy level or accelerated in its orbit. In either case, certain discrete quantities of energy are required, for when light photons have either too much or too little energy, they are not absorbed.
Electrons may orbit in pairs within an orbital, the members of the same orbital spinning in opposite directions. Most atoms at their lowest energy level (i.e., ground state) have all their electrons paired in this fashion and are said to be in the singlet (i.e., S) state. When a photon of light is absorbed and an electron is thereby raised to a higher, unoccupied orbital, it may continue to spin in the direction opposite to that of its former partner (in which case the atom is still in the S state), or it may now spin in the same direction as its former partner (in which case the atom is said to be in the triplet or T state).
In a molecule, some electrons orbit exclusively about specific atomic nuclei; others may be shared between two nuclei forming a bond (called localized or π electrons), or may orbit about several nuclei (called delocalized or π electrons). The absorption of a light photon may move a π electron to a π position. Chlorophyll is normally a singlet in its ground state. Although the absorption of light causes some x electrons to be raised to a π orbital, chlorophyll remains in a single state.
When red (680 nm) light is absorbed, an electron is raised to a higher, Sπ, orbital. Blue (430 nm) light possesses more energy per photon than red light, and its absorption raises an electron to a higher (but still Sπ) energy state. These transitions are summarized in Figure 17-11.
Once a molecule has absorbed light energy and is in an excited state, the ground state may be reestablished in three different ways:
(1) The energy may be reemitted in the form of radiation of longer wavelength (i.e., fluorescence);
(2) The energy may be converted to heat; and
(3) The excited molecule may transfer its excess energy to another molecule.
The transfer of energy from one molecule to another often involves the exchange of a high-energy electron for one of lower energy. Referring to Figure 17-11, the blue light absorbed by chlorophyll raises an electron to the Sbπ state. By losing energy in the form of heat, that electron could “drop” back to the Saπ state. The electron could then drop back from the Saπ state to the Sπ state (i.e., the ground state) by the immediate loss of energy as heat or fluorescence.
Another possibility also exists, for the electron could drop from the Saπ state to a triplet (i.e., Tπ) state by heat loss and then to the ground state by either phosphorescence (delayed fluorescence) or heat loss. Concentrated solutions of extracted chlorophyll will strongly fluoresce red when placed in a beam of sunlight or ultraviolet light.
Finally, it should also be noted that if the chlorophyll molecule is sufficiently excited, it may not return to the ground state by heat loss or reradiation; instead, the excited electron may be transferred to another molecule, leaving the chlorophyll in a temporary, oxidized state.
Primary Photochemical Events in Photosynthesis:
As shown in Figure 17-11, chlorophyll absorbs both blue and red light, and this raises electrons to Sbπ or Saπ states. The return of an electron from the Sbπ state to the Saπ state is extremely fast (about 10-12 seconds) and does not afford an opportunity for the energy to be lost by fluorescence or by transfer to another molecule. Consequently, the energy is lost as heat.
The decay of an electron from the Saπ state does permit the transfer of energy to another molecule, and this is the event that initiates photosynthesis. Consequently, a photon of red light is just as effective in initiating photosynthesis as a photon of blue light, even though the former is much less energetic.
The chloroplast contains many different pigment molecules (other chlorophylls, carotenoids, phycobilins, etc.) and the electrons of these molecules may be excited to various energy states by the absorption of light. As these excited accessory pigment molecules return to the ground state, the resulting energy is transferred to chlorophyll a molecules, causing their excitation. Because the chlorophyll a molecules present in a thylakoid vary in their absorption maxima, varying quantities of energy are required to raise their electrons to the Saπ state. The molecule that requires the least amount of energy is believed to be a pigment that absorbs long, red wavelengths, namely, the P700 molecule. (Pigments may be identified by a letter [e.g., “P”] followed by a numerical subscript.
The subscript indicates the wavelength of the light absorbed by the pigment.) It seems reasonable that light energy captured by the accessory pigments and transferred to chlorophyll a is in turn transferred from the latter to P700. Each accessory pigment or chlorophyll a molecule can only pass its energy on to pigments having absorption maxima of longer wavelength because these require less energy to be activated to the Saπ state. Because P700 has its absorption maximum at the longest wavelength, it serves as the final energy trap in a part of the photo- synthetic unit called a reaction center. There are two types of reaction centers, each with an ultimate energy-trapping chlorophyll a molecule. One is P700 and the other is P680.
Two Photosystems:
When some plants are exposed to light containing only wavelengths of 690 nm or longer, photosynthetic efficiency decreases. The effect is called the red drop (Fig. 17-12). Because the absorbed energy is funneled to P700, it would be expected that the absorption of light by the accessory pigments, chlorophyll a, or even P700 should be equally efficient. The efficiency can be increased through the addition of shorter-wavelength radiations.
This enhancement phenomenon can increase the photosynthetic rate 30 to 40% above the rate obtained by either the short wavelength or long wavelength alone. The synergistic effect of the two different wavelengths led early investigators to conclude that two distinct, photochemical reactions exist.
In one of these (called photosystem I, PS-I), P700 serves as the ultimate energy trap or reaction center, in the other (called photosystem II, PS-II), the ultimate reaction center is a chlorophyll that has its absorption maximum at 680 nm and is called P680. Within photosystems I and II, the organization of auxiliary pigments and chlorophylls form antenna complexes. These complexes act to direct light energy to the reaction center molecules P700 and P680.
In higher plants, photosystem I is a unit containing several hundred molecules of chlorophyll (mostly chlorophyll a), about 50 carotenoids, one cytochrome f, one plastocyanin, two cytochrome 6564 molecules, one or two ferredoxin molecules, and one molecule or a dimer of chlorophyll P700. Photosystem II has about 200 molecules of chlorophylls a and b absorbing light at a wavelength that is less than 680 nm, 50 carotenoids, one P680 molecule or dimer (the primary electron donor), a primary electron acceptor that is a quinone, four plastoquinones, six manganese atoms, and two cytochrome 6559 molecules.
Sequence of Energy (Electron) Flow:
The absorption of light by chlorophyll P700 or P680 alters the state of the orbiting electrons, and if the energy is not lost by reradiation or heat, the excited electrons can be transferred to another compound. Such an electron loss “bleaches” the chlorophyll and leaves it in an oxidized state. Oxidized P700 may be reduced by absorbing an electron from photosystem II, whereas the reduction of the oxidized chlorophyll of photosystem II is brought about by the oxidation of water (Fig. 17-13).
In 1938, R. Hill demonstrated that isolated chloroplasts exposed to light could evolve oxygen and reduce a variety of compounds without consuming carbon dioxide. Three years later S. Ruben and M. Kamen, using the isotope 180, were able to show that the oxygen liberated during whole plant photosynthesis was derived from water molecules, that is,
The method by which the water molecules are split is unknown, although four water molecules are required for the evolution of one oxygen molecule and four quanta of light are necessary:
Protein-bound Mn2+ and CI” may also be required. Some of these reactions are summarized in Figure 17-13.
Redox Reactions:
Electrons that are released from chlorophylls P700 and Peso pass from one to another of a series of electron transfer molecules in the lamellae. Each of these molecules has a specific electrical potential called a redox potential. The relative values of the redox potentials of the molecules involved in the transfer of electrons determine the order in which each molecule participates in the transfer.
During electron transfer, the molecules accept electrons from less positive (more negative) molecules and donate electrons to more positive (less negative) molecules. When a molecule accepts electrons, it is said to be reduced, and when electrons are given up, the molecule is said to be oxidized. The transfer of electrons following the absorption of light energy by chlorophyll therefore involves a sequence of oxidation-reduction reactions. This sequence is shown in Figure 17-14, which also identifies the intermediate electron acceptors and their redox potentials.
Photosystem I is located in the membranes of both the grana thylakoids and stroma thylakoids, whereas photosystem II is found only in the membranes of the grana thylakoids. Therefore, photosystem I functions independently in the stroma thylakoids but functions in conjunction with photosystem II in the grana thylakoids.
Absorption of light energy by P700 of photosystem I causes the loss of electrons first to an intermediate iron-sulfur protein and then to a molecule of ferredoxin imbedded in the thylakoid membrane (i.e., “bound ferredoxin” in Fig. 17-14). Bound ferredoxin is reoxidized by the transfer of electrons either to a soluble ferredoxin bound to the FAD-containing enzyme ferredoxin-NADP reductase or to cytochrome b563 (see below). Ferredoxin-NADP reductase transfers the electrons to FAD, which also accepts H + (from water) and reduces the NADP +, that is,
NADP+ + 2 (H + + e–) → NADPH + H+
The NADPH and H + spill into the stroma, where the NADPH is reoxidized during the dark reactions of photosynthesis.
In the alternative pathway leading to the reoxida- tion of bound ferredoxin, electrons are transferred to cytochrome b563. Cytochrome b563 initiates a sequence of redox reactions, passing the electrons on to cytochrome f (also called cytochrome b552), plastocyanin (PC), and ultimately to P700, completing a cycle. This cyclic set of redox reactions is coupled to the phosphorylation of ADP (cyclic photophosphorylation, described below).
In the grana lamellae the P700 oxidized by the absorption of light can be reduced by the cyclic return of the electrons from ferredoxin or by the transfer of electrons from photosystem II. Light energy absorbed by the accessory pigments of the reaction center of photosystem II is ultimately transferred to chlorophyll P680 (Fig. 17-14). The ejected electrons are absorbed by an electron acceptor called “Q,” whose identity is still uncertain but which is believed to be plastoquinone (PQ).
This photoevent initiates another set of redox reactions in which the electrons pass from Q to PQ, cytochrome b559, cytochrome /, plastocyanin, and ultimately to P700. The electrons in photosystem II do not cycle back to chlorophyll P680 but their transfer through the redox reactions to P700 is coupled to phosphorylation of ADP (noncyclic photophosphorylation, described below).
The reduction of the oxidized P680 chlorophyll is brought about by the oxidation of water via an enzyme system that is closely associated with the structure of the thylakoid and that also produces molecular oxygen. The enzyme system presumably processes four protons simultaneously to produce 02.
2H2O → O2 + 4H+ +4e–
Mn2+ is known to be a cofactor in the system. The electrons produced act to reduce the light-oxidized P680, and the H + forms a pool available for the reduction of NADP+
Cyclic and Noncyclic Photophosphorylation:
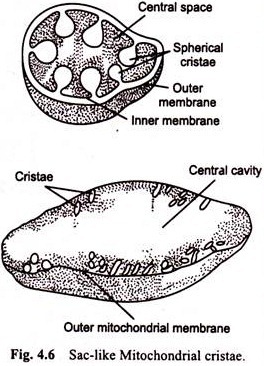
Phosphorylation of ADP occurs in chloroplasts during the light reactions and is called photophosphorylation. The photophosphorylation occurs in the lamellae of the stroma and grana thylakoids as a part of both photosystems I and II. There is a clear similarity between the mechanism of photophosphorylation in chloroplasts and electron transport system phosphorylation in mitochondria.
In both organelles, the mechanism is closely associated with a membrane and with a compartment separated from the rest of the organelle. In the chloroplast, the compartment is the loculus of the thylakoid; in the mitochondrion, it is the inner membrane-encapsulated matrix. In both organelles, the phosphorylation that occurs is coupled to electron transport through a sequence of redox reactions. Several of the molecules that participate in the redox reactions are similar, including the quinones and cytochromes.
Cyclic photophosphorylation occurs when electrons released from P700 following the absorption of light energy are cycled back to P700 “through ferredoxin, cytochrome b563, cytochrome f, and plastocyanin. The energy from these exergonic redox reactions is coupled to phosphorylation.
Noncyclic photophosphorylation occurs when electrons released from P680 of photosystem II are shuttled via plastoquinone, cytochrome f, and plastocyanin to P700 of photosystem I. The energy from these noncyclic exergonic redox reactions is also coupled to phosphorylation.
At the present time, the mechanism of coupling is not completely understood, although much evidence supports the concept that a proton gradient generated across the thylakoid membrane supports chemiosmotic coupling for mitochondria and illustrated in Figure 17-15.
Chloroplasts illuminated in an un-buffered medium quickly cause the medium to become alkaline, implying that protons are transported from the medium into the thylakoid. In darkness the system reequilibrates. If the medium is made alkaline, phosphorylation of ADP occurs in the dark. Compounds that degrade the thylakoid membrane (such as detergents) also cause leakage of protons from the thylakoid and prevent phosphorylation.
The standard free energy change that takes place when ATP is formed from ADP and Pi is about 30.5 kJ/mole (7.3 kcal/mole); this is equivalent to a redox potential of about 0.45 to 0.61V. Most of the measurements that have been made indicate that one molecule of ATP is produced for each pair of electrons moved from photosystem II to photosystem I (i.e., non- cyclic photophosphorylation).
Several recent studies strongly suggest that more than one molecule of ATP is produced by the reactions that begin with the splitting of water (i.e., photolysis) and end with the reduction of NADP+. The second ADP phosphorylation is believed to be coupled to the photolysis. If the number of ATP molecules produced during the light reactions is to balance the number consumed by the dark reactions, then three ATP must be produced for each pair of electrons transported from H2O to NADP+. The third ATP may be generated by cyclic photophosphorylation.
Another light-induced phosphorylation of ADP called pseudocyclic photophosphorylation occurs when reduced ferredoxin transfers electrons through a series of reactions to O2 (not to NADP+ or P700), with the result that water and ATP are formed.
Summary of the Light Reactions:
Two photosystems function during the light reactions of photosynthesis. As each system absorbs four quanta of light energy, chlorophylls P680 (photosystem II) and P700 (photosystem I) are excited, releasing two pairs of electrons to acceptor molecules. Chlorophyll P680 is returned to its reduced state by the oxidation of two water molecules, and in the process one molecule of oxygen is evolved. P700 is returned to its reduced state by electrons that are derived from photosystem II.
The transfer of two pairs of electrons from water through photosystem II to photosystem I is coupled to the photosphosphorylation of two ADP molecules, thereby producing two molecules of ATP and two molecules of water. The two pairs of electrons released from P700 of photosystem I reduce two NADP + forming two NADPH and two H+. Therefore, the net result is
The two ATP and two NADPH molecules produced by the light reactions occurring in the grana lamellae are used in the synthetic (dark) reactions that take place in the stroma. The latter reactions fix C02 into sugars. For each CO2 fixed, two NADPH (and two H+) and three ATP molecules are required (see below). Where the third ATP molecule is formed is not yet clear; it may be obtained from cyclic photophosphorylation or pseudocyclic photophosphorylation.