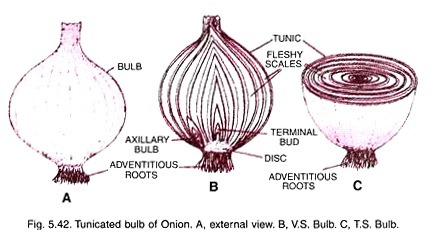
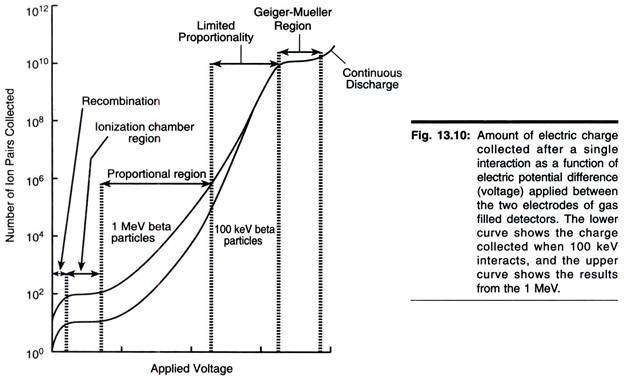
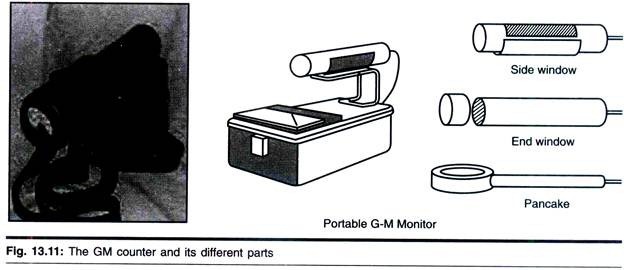
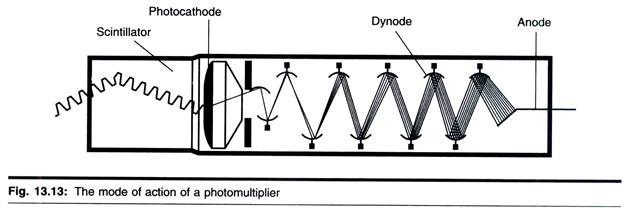
The size, shape, and structural organization of mitochondria, as well as the number of these organelles per cell and their intracellular location, vary considerably depending on the organism, tissue, and physiological state of the cell examined.
Some cells, usually unicellular organisms, contain a single mitochondrion.
Figure 16-3 contains a photomicrograph of the single mitochondrion in the motile swarm spore of Blastocladiella emersonia, a fungus, and a model of the single mitochondrion of Chlorella fusca, an alga.
At the other extreme are cells such as Chaos chaos, an amoeba, which contains several hundred thousand mitochondria. Cells of higher animals also contain various numbers of mitochondria. Sperm cells have fewer than 100 mitochondria. Kidney cells generally contain less that 1000 and liver cells can contain several thousand. Prokaryotic cells such as bacteria and blue-green algae do not contain mitochondria. The functions associated with mitochondria are carried out in the cytosol or are associated with the plasma membrane.
The distribution of the mitochondria in the cell can change with time. In Blastocladiella (Fig. 16-3), the single mitochondrion is at the base of the flagellum. Mitochondria also appear to be concentrated in metabolically active areas of cells. In epithelial cells lining the lumen of the small intestine, the mitochondria occur in greater numbers near the cell surface that is adjacent to the lumen (where active absorption of diestive products is occurring).
In general, when mitochondria are present in greater numbers in one part of the cell than another, it is usually near a site where significant ATP utilization is occurring. For example, in muscle tissue the mitochondria are aligned in rows parallel to the contractile fibrils (Fig. 16-4). In many plant cells, cyclosis, the active streaming of the cytoplasm about the cells, tends to distribute the mitochondria uniformly.
 The number and distribution of mitochondria in a cell are closely related to the activity of the cell and its organelles. Cells that are actively growing, producing especially large’ amounts of some product such as digestive enzymes, actively transporting materials into the cell, or undergoing movement may display increased numbers of mitochondria during periods of activity and reduced numbers during periods of quiescence.
The number and distribution of mitochondria in a cell are closely related to the activity of the cell and its organelles. Cells that are actively growing, producing especially large’ amounts of some product such as digestive enzymes, actively transporting materials into the cell, or undergoing movement may display increased numbers of mitochondria during periods of activity and reduced numbers during periods of quiescence.
In yeast cells grown anaerobically, successive generations contain fewer and fewer mitochondria. However, cells that have been cultured in the absence of oxygen rapidly produce greater numbers of mitochondria if oxygen and appropriate nutrients are added to the culture. An increased rate of cell growth and division also occurs since greater numbers of mitochondria produce more ATP in order to facilitate absorption of nutrients and the synthesis of cell components.
The size and shape of mitochondria, like the number in a cell, vary from one tissue to another and with the physiological state of the cells. Most mitochondria are ovoid bodies having a diameter between 0.5 and 1.0 µm and a length up to 7 µm.
Usually the lower the numbers of mitochondria per cell, the larger are the individual organelles. In many electron photomicrographs, mitochondria appear to be dumbbell-shaped or racket-shaped. These odd shapes may be a reflection of the fission process by which mitochondria are believed to proliferate. Dumbbell shapes are seen just prior to separation and the “handles” (or “tails”) of racket-shaped mitochondria may be the bridges that connect the separating mitochondrial halves (Fig. 16-5).
From the point of view of fine structure, mitochondria are especially interesting and very intricate organelles. Because mitochondria are so small, light microscopy reveals little about their structure. The contemporary model of mitochondrial architecture is therefore based on decades of study using the transmission electron microscope.
Figure 16-6 illustrates the typical organization revealed in thin sections of these organelles. Recently, some rather spectacular and highly informative photomicrographs of mitochondrial structure have been obtained by scanning electron microscopy of organelles that have been cracked open (Fig. 16-7).
In the past, SEM has not offered the degree of resolution needed to reveal organelle substructure. However, using a special fracturing technique and a novel high-resolution SEM, K. Tanaka, Y. Masunaga, and T. Naguro have obtained detailed photomicrographs of mitochondria and a number of other organelles. After tissue fixation in OsO4, the samples are frozen, cracked open, and then treated with dimethylsulfoxide (DMSO).
Following a second treatment with osmium, followed by dehydration with ethanol and critical point drying, the fine structure is revealed in bold relief. The three- dimensional effect is striking and amazingly consistent with three-dimensional models formulated on the basis of the earlier TEM studies. Because mitochondria from diverse sources exhibit certain features in common, a “generalized” organelle may be described (Fig. 16-8).
The mitochondrion is enclosed by two distinct membranes called the outer and inner membranes (Figs. 16-6 and 16-7). The inner membrane separates the organelle’s volume into two phases: the matrix, which is a gel-like fluid enclosed by the inner membrane and the fluid-filled in- termembrane space between the inner and outer membranes.
The matrix and inter membrane spaces as well as the outer and inner membranes themselves contain a variety of enzymes (Table 16-1). The matrix contains a number of the enzymes of the Krebs cycle (tricarboxylic acid cycle, or TCA cycle) as well as salts and water. Suspended in the matrix are strands of circular DNA (Fig. 16-9) and ribosomes.
A number of other inclusions have been observed in the mitochondrial matrix of diverse kinds of cells. These include filaments and tubules, what appear to be crystalline protein inclusions, and a number of small granules. The inter membrane space contains some enzymes (Table 16-1), but generally is devoid of particulate inclusions.
The Mitochondrial Membranes:
The inner and outer membranes are distinctly different in structure and function. Although accurate measurements of the thicknesses of the membranes by electron microscopy are difficult because various fixatives promote different degrees of swelling, the inner membrane appears to be somewhat thicker (6.0-8.0 nm) than the outer membrane (about 6.0 nm).
The inner membrane has a greater surface area because it possesses folds that extend into the matrix. These projections, called cristae, vary in number and shape. With distinct exceptions, the cristae of mitochondria in higher animal cells may almost bridge the matrix. Usually the cristae lie parallel to one another across the long axis of the mitochondrion but in some cells they run longitudinally or form a branching network (Fig. 16-10).
In protozoa and many plants, the cristae form a set of tubes that project into the matrix from all sides, sometimes twisting in different directions. The number of cristae may increase or decrease depending on the level of aerobic activity. Active aerobic tissue cells producing large amounts of ATP generally contain mitochondria with extensive cristae.
The organization of protein and lipid in the outer and inner mitochondrial membranes has been the subject of intense study for many years. Chemically, the two membranes are qualitatively and quantitatively distinct, differing from one another and also from other intracellular membranes.
The inner membrane is much richer in protein than the outer membrane and the proteins themselves are more deeply embedded in the membrane. The outer membrane contains three or four times more phospholipid than the inner membrane and contains most of the membrane cholesterol. In contrast, the inner membrane is rich in cardiolipin. Differences in the two membranes are also apparent in freeze-fracture views of the E and P faces (Fig. 16-11).
The P fracture face (PF) of the outer membrane contains more than three times as many particles as the E fracture face (EF). In contrast, the EF of the inner membrane contains almost as many particles as the PF of the outer membrane, whereas the inner membrane’s PF contains twice as many particles as its EF.
This is not surprising in view of the manifold enzymatic activities of the metrical surface of the inner membrane (see below). The organization of protein and lipid in the inner membrane is depicted in Figure 16-12). Much of the protein of the inner membrane can be stripped away using very dilute solutions of acetic acid, suggesting that a large proportion of the inner membrane proteins are extrinsic. Attached to the matrix side of the inner membrane are many spherical particles 8.0-9.0 nm in diameter.
These inner membrane spheres, first described by Fernandez-Moran in 1962, are seen in the scanning electron photomicrographs of Figure 16-7 and the negatively stained preparation shown in Figure 16-13. They appear to be spaced regularly along the membrane and are borne on stalks. These inner membrane spheres have been identified as the primary sites of oxidative phosphorylation.
Each crista is formed from two inwardly projecting leaflets of the inner mitochondrial membrane. Fine structural studies by F. Sjostrand using a freeze- fracture technique suggest that little or no space exists between these two layers (Fig. 16-14).
Instead these appear to form a single structure containing large globular proteins about 15 nm in diameter and small quantities of lipid at the matrical surfaces. Sjostrand also contends that the inwardly projecting cristael membrane leaflets are anchored to the inner membrane by one or more stalks or peduncles rather than arising as shelflike folds (Fig. 16-15). The scanning electron photomicrographs of Figure 16-7 reveal little space between the two cristael membranes and also suggest pointlike attachments of the cristae to the inner membrane.
As noted earlier, mitochondria contain some DNA, but the amount is sufficient to encode only a small number of mitochondrial proteins. Nearly all mitochondrial proteins are encoded by nuclear DNA. These proteins are synthesized by cytoplasmic ribosomes and then imported into the four mitochondrial compartments (i.e., outer membrane, inner membrane, inter membrane space, and matrix).
Like secretory and plasma membrane proteins, proteins destined for the inner mitochondrial membrane or the matrix are synthesized with a transient leader sequence; their temporary anchorage to the outer membrane and their subsequent passage through it are assisted by a small protein present in the cytosol.
After import, the leader sequence is enzymatically removed by a protease present in the mitochondrial matrix. Proteins destined for the outer mitochondrial membrane do not contain a leader sequence. Targeting and anchorage in the outer membrane is specified by the amino acid sequence in the proteins’ N-terminal region. Import of proteins of the inter membrane space (e.g., cytochrome c) does not require a leader sequence but may involve a preliminary interaction with specific receptor proteins in the outer membrane.
In addition to their chemical and structural differences, the outer and inner membranes differ significantly in permeability. The outer membrane is permeable to a wide variety of substances of a molecular weight up to about 5000. When the fluid from the inter membrane space is isolated, it reflects the water- soluble low-molecular-weight components of the cytosol. In contrast, the inner membrane has a limited permeability especially to substances with molecular weights above 100 to 150.
The difference in permeability of the two membranes can be used to advantage to separate the outer membrane from the inner. Although several variations of the method exist, the basic procedure is to disrupt the cells and isolate intact mitochondria by differential or density gradient centrifugation. The mitochondria are then placed in a hypotonic solution, causing them to swell. Usually, a dilute phosphate buffer solution is used for this purpose. As the mitochondria swell, their outer membranes rupture and fragment; the inner membranes also swell, causing a loss of organized cristael structure, but the inner membrane does not break.
The mitochondria are then transferred to a hypertonic solution, causing the matrix to shrink, pulling the inner membrane away from the outer membrane. The hypertonic solution is frequently a sucrose solution containing ATP and Mg2+.
Because the inner membrane may be attached to the outer membrane at several points (probably by proteinaceous connecting strands), some workers use EDTA or signification to aid the separation of the membranes. Resuspension in an isotonic solution allows the matrix to reassume its normal size and typical morphology. Isolation of the separated membrane fractions can be achieved by centrifugation. The results of a typical isolation are shown in Figure 16-16.
Conformational States of Mitochondria:
Changes in the level or type of physiological activity occurring in mitochondria can be related to differences in the morphological appearance of the organelles. Accordingly, C. R. Hackenbroek has shown that the orthodox conformational state (Fig. 16-17) of mitochondria (the appearance most commonly seen in photomicrographs) is typical of inactive organelles.
The condensed conformational state (Fig. 16-17) corresponds to periods in which phosphorylation of ADP to form ATP and electron transport occur at high rates. In the condensed conformational state, the cristae are more randomly distributed and the inter membrane space is greatly enlarged. The transition from the orthodox to the condensed conformational state is triggered by the binding of ADP to ADP-ATP translocase molecules in the inner membrane.
Over the years, numerous investigators have studied the relationship between the structural organization of the mitochondria and its specialized metabolic functions. As seen in Table 16-1, the location of many enzymes has been determined, and it is generally possible to assign specific functions to the outer membrane, inter membrane space, the inner membrane, and the matrix. In some cases, the enzyme activity can be assigned to a specific surface of the membrane (e.g., the ATP-synthesizing enzymes and succinate dehydrogenase are in the inner membrane).
Among the most thoroughly studied processes unique to mitochondria are substrate oxidation, respiratory chain oxidation-reductions, and oxidative phosphorylation (Fig. 16-18). Products of metabolic reactions in the cytosol (such as pyruvate formation during glycolysis) enter the mitochondrion to be oxidized by the Krebs or tricarboxylic acid cycle enzymes (Fig. 16-19). The enzymes that catalyze these reactions (except succinic dehydrogenase, see below) are believed to be localized in the matrix or are easily removed from the surface of the inner membrane that faces the matrix.
As a result of the Krebs cycle oxidations, CO2 and water are formed as end products and a number of special oxidation-reduction compounds are reduced These compounds subsequently participate in the initial step of a sequence of oxidation and reduction reactions called the respiratory or electron transport chain that is specifically associated with the inner membrane of the mitochondrion. The result of this chain of reactions is the reduction of O2 to form H2O. A third major process called oxidative phosphorylation is coupled to the respiratory chain and brings about the conversion of ADP to ATP. Oxidative phosphorylation is intimately associated with the inner membrane spheres.
In effect, pyruvate and other small molecules produced during metabolism in the cytosol cross the permeable outer mitochondrial membrane and the inter-membrane space. It is on entering the inner membrane that the three major reaction sequences (Krebs or TCA cycle, respiratory chain oxidation-reductions, and oxidative phosphorylation) begin.
Pyridine nucleotides that are reduced during reactions in the cytosol (e.g., NADH produced in the glycolytic pathway and NADPH produced in the pentose phosphate pathway;) may also permeate the outer mitochondrial membrane, ultimately transferring their reductive capacity to the respiratory chain.