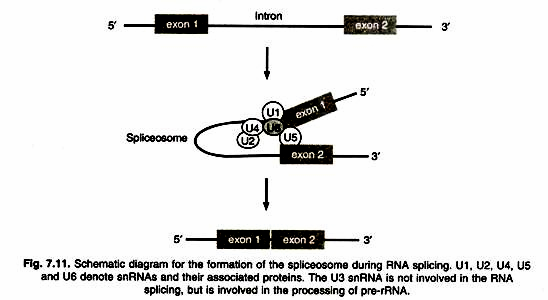
A consistent observation made for plasma membranes that was not explained by the bimolecular lipid leaflet model was the very low surface tension of the cell membrane.
In 1935, J. F. Danielli and E. N. Harvey proposed that oil droplets and other lipid inclusions in cells were bounded at their surfaces by an organized layer of the lipid and a layer of protein.
It was postulated that the protein, which consisted of a monomolecular layer of hydrated molecules, faced the aqueous cytoplasm and simultaneously interacted with the polar portions of the lipid layer.
The nonpolar portions of the lipid layer faced the hydrophobic oil phase of the droplet interior (Fig. 15-4).
In this structure, the natural surface activity of the protein would account for the low interfacial tension of the droplet membrane. Shortly thereafter, Danielli and H. Davson suggested that the plasma membrane itself might be composed of two such lipid-protein bilayers—one facing the interior of the cell and the other facing the external milieu (Fig. 15-5).
In this arrangement, the association between the surface proteins and bimolecular lipid leaflet would be maintained primarily by electrostatic interactions between the polar ends of each lipid molecule and charged amino acid side chains of the polypeptide layers.
Either electrostatic or van der Waals bonds could bind other groups to the outer protein surface. Danielli and Davson proposed that such a membrane would exhibit selective permeability, being capable of distinguishing between molecules of different size and solubility properties and also between ions of different charge.
By the early 1950s, several modifications were made in the Danielli-Davson membrane model. For example, it was suggested that glycoproteins might be adsorbed to the outer membrane surface, thereby accounting for the antigenic properties of cell membranes. Pores through which certain materials are exchanged between a cell and its environment were presumed to exist, the channels formed by periodic continuities (bridges) between the outer and inner protein layers.