Introduction:
Predation is an interaction between two different species and may be defined as the consumption of all or part of another individual (the prey).
According to C.J.Krebs (1994), five specific types of predation may be distinguished:
(a) Herbivores are animals that prey on green plants and often the plants are not killed but may be damaged;
(b) Carnivores are typical predators, preying on herbivores or other carnivores;
(c) Parasites are animals or plants that depend on the host for nutrition;
(d) Insect parasitoids are another type of predator, which lay eggs on or near the host insect which is subsequently killed and eaten; and
(e) Cannibalism is a special form of predation in which the predator and prey belong to the same species.
Predators which play a crucial role in maintaining the structure of the community, for example star fish on the sea coast and tiger in the forest, are called keystone predators (Paine, 1966; Hunter and Price, 1992). Removal of a keystone species allows spread of some other organisms which may in turn crowd over an area reducing the diversity and complexity of local food webs.
Predation is an important ecological process from the following three points of view. First, predation may restrict distribution or reduce abundance of the prey. If the prey is a pest, we may think the predation useful. However, if the effected animal is a valuable resource, we may think the predation undesirable.
Second, like competition, predation influences species diversity in a community. Third, predation is a major selective force, and many adaptations, such as warning colouration, may be explained on the basis of predator- prey co-evolution. There are many cases of co-evolution of predator and prey, which is the ecological basis for biological control of undesirable species.
The predators have evolved to become more efficient at searching and capturing its prey, and prey species in response have evolved to become more efficient at evading capture by the prey. The mechanisms underlying the dynamics of predator and prey populations are best understood by studying predator- prey interactions at the individual level.
Predator – Prey Interaction:
The act of predation can be described into sequential stages of search, pursuit, attack, capture and ingestion, and each stage calls for decision making by the predator. Prey capture success depends on various parameters relating to the predator (body size, mouth size, searching efficiency), prey (body size, evasive behaviour, visibility) and the ambient medium (light intensity, turbidity). There are many examples of evolutionary compromise in body size of the prey species. For example, In case of Daphnia (prey) eaten by copepods (predator), large size of prey is helpful to avoid predation; but in case of Daphnia eaten by fish, small size of prey is helpful to avoid predation.
Prey Profitability:
The profitability of a prey to the predator is a ratio of its energy content and the handling time. Given a choice between two potential prey types, a predator, which is optimizing its effort, should choose the most profitable prey. Suppose in one case it is 100/5 sec and in the other case it is 100/15 sec., the predator would like to capture the first prey for obvious reasons. Optimal foraging models try to determine in which patch a predator should forage, how long he should spend foraging in a given patch, and what prey species he should include in his diet, to maximize net energy intake.
According to optimal foraging theory (Stephens and Krebs, 1986), the ability of predators to aggregate in patches of high prey density is a critical element in determining how effective the predator can be at limiting prey populations. The ideal free distribution theory suggests that predators should move among sites until profitability is equal at all sites.
If profitability was higher at one site, the theory predicts that predators would naturally redistribute themselves. This assumes that predators are ideal in their assessment of the quality of patches, and free to move from patch to patch. Many experimental demonstrations of this process have been performed. In one case six stickleback fish were placed in a tank and food added at both ends, with one end offering low food density and the other high food density. The distribution and movement of the fish reflected accurately the variation in the food density.
Predation-Deterrent Adaptations:
Prey species have evolved many predation-deterrent adaptations, which are morphological (increased transparency to elude detection by visual predators, changes in body size, coloniality, spination), chemical (toxic chemical secretion, unpalatably) and behavioural (evasiveness, diel vertical migration). Many of these defenses are readily inducible by the predator through info chemicals called kairomones. The prey also responds to predation pressures by adaptive changes in life history traits such as age at first reproduction, frequency of reproduction, clutch size and longevity.
Predator’s Response to Changes in Prey Population Density:
The response of a predator to changes in population density of its prey is of considerable relevance in evaluating the biological control potential of a predator. In a simple one predator- one prey system, there are four possible responses of a predator to an increase in prey population density.
These are:
(a) A numerical response, in which the density of predators increases by reproduction in a given area;
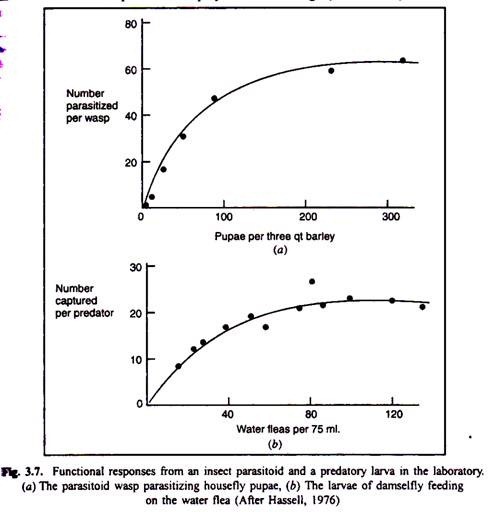
(b) A functional response, (Holling, 1959), in which the number of prey eaten by individual predators changes (Fig. 3.7);
(c) An aggressive response, in which individual predators move into and concentrate in certain places within the study area; and
(d) A developmental response, in which individual predators eat more or less prey as predators gradually become mature ( Murdoch, 1971).
If we can measure the functional, numerical, developmental and aggressive responses for a predator- prey system, we can easily determine the total response of the predator’s ell, 1981). If the total response is sigmoid (Fig. 3.8), the predator may limit the density of r prey at low prey densities. An important question is whether mortality caused by the predators increases with rise in prey density.
The mortality rate increases with prey density only for the first part of the sigmoid curve (up to C in Fig 3.8). At higher prey densities, the predator will exert no ailing influence on the prey because it will be swamped by prey numbers. Thus, it may be chided that predators may have important effects on prey abundance when prey populations: Vow and become unimportant when densities prey are high (Krebs, 1994).
Lotka- Volterra Predator- Prey Model:
The Lotka- Volterra predator- prey model, first presented by Lotka (1925) and Volterra (1926), a simple mathematical model representing the interaction between predators and their prey.
However, the model makes three simplifying assumptions:
(i) There is only one predator and one prey species involved in the interaction;
(ii) Prey numbers increase if the number of predators falls low a threshold, and decrease if there are more predators; and
(iii) Predator numbers increase if die number of prey rises above a threshold and decrease if there are fewer prey.
This model provides a basis for understanding how predator and prey populations sometimes come to oscillate with respect to one another. Despite the simplicity of this model, the pattern shows clear similarities to the cycling observed in the snowshoe hare-lynx example.
Lotka and Volterra expressed the rate of growth of both predator and prey populations by the following differential equations:
dP/dt = g(H, P)———– for the predator
dH/dt = f (H,P)————- for the prey
where P = predator population size
H = prey population size
f and g = arbitrary functions of variables H and P
Thus, the rate of growth of prey population, dH/dt, is some joint function (f) of the size of prey population (H) and the size of predator population (P). It may be recalled that the general form of these models is the same as that of the population growth models in which the change in population size (dN/dt) was shown as some function (f) of N,f (N). The Lotka – Volterra predator- prey model is a simple elaboration of this idea. What has been included in the functions (H, P) and g (H, P) are terms that account for how consuming and being consumed add and remove individuals from the populations of predator and prey.
Nicholson – Bailey Model:
Nicholson and Bailey (1935) have proposed a model for parasitoid- host population dynamics. The model indicates that at least two factors may lead to co-existence of parasitoid and host. First, if the birth rate (b) of the parasitoid is made to decrease as the density of the parasitoid increases, the expanding oscillations of the model will be changed to damped oscillations.
Second, we recognize that as the parasitoid population density increases, the efficiency with which each parasitoid finds un-attacked hosts, a, will decrease. If either of these enhancements is included in the model a stable interaction is predicted. Thus, in these models the rate of prey removal was an asymptotic function of the predator density. The model predicts that parasitoid-host interactions will be instable because they will undergo ever-increasing oscillations (Ricklefs and Miller, 2000)
Predation-Sensitive Food Hypothesis:
A model of prey-predator interaction where the predator population is less strongly dependent on prey levels, prey density is held near its carrying capacity and predation is more likely to be influenced by risky behaviour on the part of the prey, is called predation- sensitive food hypothesis (Sih, 1982; Sih and More, 1990; Abrams, 1991). The hypothesis recognizes the trade-off between the need to avoid the action of the predator and the need to obtain sufficient nourishment to survive a 1 limited-food environment (Ricklefs and Miller, 2000).
The hypothesis emphasizes the ideas that are predation may not be the only factor operating to limit prey population size; intraspecific competition during times of food limitation may also play an important role (Sinclair and Arcese, 1995).
Plant Defense from Predation:
Plants defend themselves from predation in two ways:
(i) Defensive structures, and
(ii) Toxicity and un-palatability.
Defensive structures exist in a variety of forms from simple small hairs on the of surface, which may frap insects and other invertebrates, to large hooks, barbs and spines (e.g., Utrica dioca, Rosa canina and Acacia spp.) which deter mammalian herbivores. The size and prevalence of such defensive structures may also be induced in defoliated plants.
In plants, there is a vast variety of chemical compounds used to defend plants against predators and parasites. These secondary compounds may either be directly toxic (e.g., mustard oils in cabbages, nicotine in tobacco, cyanide in white clover, cardiac glycosides in milkweeds) or they reduce the food value of the plant, for example, by reducing the availability of the leaf tissue protein to the animal gut.
The tannins in the mature leaves of many woody plants bind to proteins, thus making them inaccessible to the predator’s gut. Similarly, tomato plants produce protease inhibitors, which inhibit the protease enzymes in the herbivore’s gut.