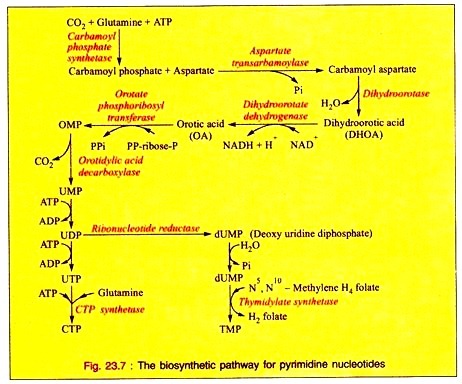
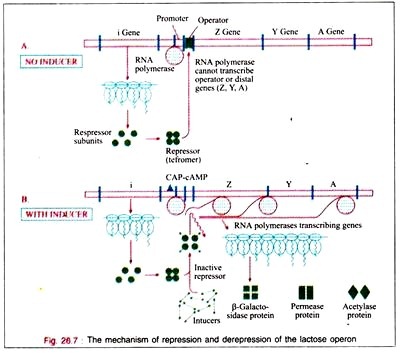
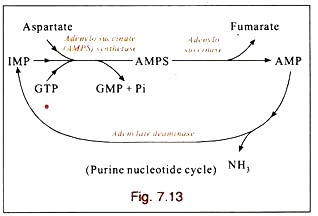
Vertebrates possess three types of muscle tissue:
(1) Smooth muscle, the contractile elements of most of the digestive system and most visceral organs;
(2) Cardiac muscle, found only in the heart; and
(3) Striated (or skeletal) muscle, responsible for most of the gross movements of the body, especially the skeleton.
Each of these tissues is composed of cells called muscle fibers, which contain cytoplasmic filaments of actin, myosin, and other proteins responsible for the contractile nature of muscle. The filaments are arranged differently in each of the tissue types. The arrangement is most highly organized in striated muscle, and it is this type of muscle tissue that has been most extensively studied.
The organization of striated muscle is shown diagrammatically in Figure 24-10. During embryonic development, the muscle fiber is formed by the end-to- end fusion of many ceils into a continuous tube-like structure.
This explains why striated muscle fibers are such long cells and are multinucleate. The plasma membrane of the muscle fiber is called the sarcolemma; in addition to its exceptional length, the sarcolemma is characterized by numerous pore like invaginations that extend into the sarcoplasm (cytoplasm) at right angles to the long axis of the cell.
These transverse or T system extensions of the sarcolemma (Fig. 24-11) make contact with most of the internal myofibrils—the contractile units of the cell. Each myofibril contains a large number of thick (15-nm diameter) and thin (7-nm diameter) filaments called myofilaments that run parallel to each other and to the long axis of the cell. Seen in cross section (24-12), the filaments are arranged in a repeating geometric pattern. The thick filaments are equidistant from each other with each surrounded by six thin filaments in a hexagonal array (Fig. 24-12b).
During the contraction of the cell, the thick and thin filaments slide past one another. When examined microscopically in longitudinal section, each myofibril reveals areas of different density (Fig. 24-13). The alternating light and dark areas are respectively called the I (i.e., isotropic) and A (i.e., anisotropic) bands. At the center of each I band there is a dark line called a Z line, and those portions of a myofibril that extend from one Z line to the next are termed sarcomeres.
One end of each thin filament is anchored in the Z line and the other end projects toward the center of the sarcomere. The thick filaments are sandwiched between the thin filaments but are not attached to the Z lines. Within each sarcomere, the A bands extend from one end of a stack of thick filaments to the other.
As seen in Figures 24-12 and 24-13, there is a region within each A band that is devoid of actin filaments; this region appears somewhat less dense than the remainder of the A band and is called an H zone. Finally, at the center of the H zone is the somewhat darker M line. As contraction occurs, the thick and thin filaments slide past each other, so that the H zone disappears and the I band becomes much narrower (Fig. 24-14). The Z lines are drawn closer together and the myofibril as a whole becomes thicker.
The myosin monomers that comprise the thick filaments are fibrous proteins containing a “head” and “tail” portion (Fig. 24-15). Each myosin molecule is composed of two heavy polypeptide chains of MW 200,000 and four light chains of MW 20,000. Myosin can be cleaved into three pieces using proteolytic enzymes. Treatment with trypsin releases part of the tail (i.e., light meromyosin, LMM) from the remainder of the molecule.
Light meromyosin has no ATPase activity and cannot combine with actin. The remaining portion of the molecule, called heavy meromyosin (HMM), contains ATPase activity and binds actin. Treatment of HMM with the enzyme papain severs the head (the portion containing the ATPase activity) from the tail.
In thick filaments, the myosin molecules are arranged with their tails parallel to each other and their heads projecting away from the long axis of the filament at intervals (Fig. 24-16). No heads are present in the center of the filament, this region coinciding with the H zone. The heads of the myosin molecules form cross-bridges with adjacent actin filaments.
Two functional domains can be identified in each head. One domain contains the two cross-bridges that link the myosin to actin, and the other domain provides a flexible connection or hinge between the cross-bridges and the remainder of the myosin molecule.
Thin filaments are composed primarily of actin, but also present are small amounts of tropomyosin, troponin, α actinin, and β actinin (see Fig. 24-17). In the ionic environment of the cell, the actin exist in the fibrous (i.e., F-actin) form consisting of two chains of actin monomers coiled about each other. When extracted from muscle tissue and dialyzed, actin becomes globular (i.e., G-actin) as the monomers separate. Each actin monomer is a single polypeptide of MW 43,000. The proteins tropomyosin and troponin are arranged at intervals along the actin filament and serve as regulatory agents in contraction. Actinin may function in polymerization of the actin monomers.
Muscle Contraction:
When mixed together in solution purified myosin and actin molecules will bind to each other by forming cross-bridges. Experimentally, this is noted by an increase in the viscosity of the solution. Cross-bridges between actin and myosin filaments also exist in intact muscle fibers. It is the oar-like action of these cross-bridges accompanied by filament sliding that is the basis of contraction.
It has been known for some time that the cyclic contraction and relaxation of muscle cells is intimately tied to the metabolism of ATP. For example, when ATP and Mg2 + are added to an actin/myosin mixture, the solution’s viscosity rapidly diminishes and the ATP is hydrolyzed. This is interpreted as an ATP-elicited disengagement of the cross-bridges between actin and myosin.
The cyclic breakage and reformation of these cross-bridges accompanied by their oar like movement is believed to be the in situ source of filament sliding. The ATP hydrolysis that accompanies this action is catalyzed by an ATPase present in the head of the myosin molecule. The exact role played by ATP hydrolysis during filament sliding has been a subject of controversy for many years and still remains elusive.
A full cycle from relaxation through contraction and back to relaxation involves four phases:
(1) Triggering (or initiating) contraction,
(2) The “power stroke” (i.e., contraction per se),
(3) Relaxation, and
(4) The generation of energy for the process.
Triggering (Initiating) Contraction:
Prior to contraction the following conditions exist:
(1) The cross- bridges between the myosin heads and the actin filaments are in a weakly interacting state, with each myosin head oriented at right angles to the filament’s long axis;
(2) The ATP and Mg2 + concentrations of the sarcoplasm are high, whereas the concentration of Ca2+ is low (Fig. 24-18, step 1); and
(3) The myosin heads contain tightly bound ADP and phosphate molecules. Strong cross-bridge interaction between myosin and actin is prevented by the regulatory proteins troponin and tropomyosin.
Normally, contraction of a muscle cell is initiated when a nerve impulse causes depolarization of the sarcolemma at the myoneural junction (i.e., the junction between a muscle and nerve cell). The depolarization quickly spreads across the sarcolemma and down through the complex system of invaginations forming the sarcoplasmic reticulum or T system.
Depolarization is accompanied by a change in the permeability of the sarcoplasmic reticulum membranes to ions. Of special importance are the resulting movements of calcium ions, which are rapidly released into the sarcoplasm from storage vesicles elaborated by the sarcoplasmic reticulum. Calcium ions bind to troponin causing a conformational change in the complex and a shift in the position of tropomyosin. As a result, the binding sites on actin become available for further and stronger interaction with the myosin.
The Power Stroke:
As just noted, calcium ions entering the sarcoplasm bind to troponin, causing the troponin and tropomyosin to undergo a conformational change. As a result, a binding site on a G-actin monomer is exposed and reacts with the actin binding site of the myosin head (Fig. 24-18, step 2).
The myosin head immediately undergoes a conformational change, altering to 45° its angle to the tail of the myosin molecule. Consequently, the actin filament is caused to slide about 12 nm. This movement is the power stroke (Fig. 24-18, step 3) and occurs at almost the same moment for thousands of pairs of actin and myosin molecules, thereby positioning a new set of myosin heads and actin binding sites near one another. The process is repeated again and again, causing each actin filament to move a total distance of about 250 nm.
The transition between the 90° and 45° conformation is accompanied first by the release of the bound phosphate from the myosin head and then by the release of ADP (Fig. 24-18, step 3). It is to be noted that no ATP is consumed during the power stroke. ATP binds to the myosin head, thereby occupying the site just vacated by ADP (Fig. 24-18, step 4).
Binding of the ATP is accompanied by a transient detachment of the cross-bridges and transition back to the 90° conformation. With the myosin head once again oriented at right angles to the filament’s long axis, the ATP is hydrolyzed (Fig. 24-18, step 1). Thus, the filament system is primed for another round of sliding activity as the cycle is ready to be repeated.
Relaxation:
The cessation of nervous stimulation allows the sarcolemma of the muscle cell to reestablish its polarity and regain its normal permeability. As the membrane repolarizes, calcium ions that entered the sarcoplasm are actively transported back into vesicles of the sarcoplasmic reticulum. The transport of Ca2 + is effected by an enzyme of the sarcoplasmic reticulum (i.e., a Ca2 + -dependent ATPase); this enzyme constitutes a large proportion of the sarcoplasmic reticulum protein.
With the removal of Ca2 + from the sarcoplasm, the conformation of troponin changes so that the binding site on the actin is again shielded from the myosin head. The thick and thin filaments slide back to their former positions, their movement being passive (i.e., resulting from the natural elasticity of the muscle tissue or the effects of gravity) or as a result of the contraction of antagonistic muscles (muscles pulling in the opposite direction).
ATP Consumption during Muscle Activity:
Large amounts of ATP are consumed during muscle activity. At least two ATP molecules are consumed for each interaction between a myosin molecule and actin (i.e., there are two ATP-binding sites per myosin molecule), and one ATP is required for each calcium ion returned to the sarcoplasmic reticulum (i.e., two-thirds of ATP consumption is attributed to contraction and one- third to relaxation).
The ultimate source of ATP for the muscle fiber is the phosphorylation of ADP during cellular glycolysis and respiration. However, the immediate source is the phosphorylation of ADP using phosphocreatine (Fig. 24-19), a substance uniquely present in high concentration in muscle cells. Phosphate is rapidly transferred to ADP through the action of creatine kinase, which maintains an almost constant level of ATP in the muscle fiber during normal levels of contractile activity. (Experimentally it is possible to deplete the phosphocreatine supply by causing repeated contractions while blocking cellular glycolysis and respiration.)
The restoration of the depleted phosphocreatine is brought about first by the synthesis of ATP during glycolysis and/or oxidative respiration and then through the action of creatine kinase, which now catalyzes the transfer of phosphate in the opposite direction (Fig. 24-20).
There are two types of striated muscle tissue—red and white—and the relative amounts of ATP contributed in each tissue by glycolysis and respiration vary with each type. The red (or slow) fibers are rich in mitochondria and cytochromes and employ oxidative phosphorylation for ATP production. Their demand for oxygen is satisfied in part by the presence in these cells of large quantities of the oxygen-storing protein myoglobin. Fatty acids are a major sub strate of these cells and are converted to acetyl-CoA. Acetyl-CoA enters the Krebs cycle, producing CO; and the reducing power for oxidative phosphorylation. White fibers contain few mitochondria and little myoglobin. In these fibers, glycolysis is the primary source of ATP.
Red fibers are found in greater numoer in muscles with slow, rhythmic contraction properties such as the flight muscles of birds. They are slow contracting because they have low myosin-ATPase activity. They resist fatigue because ATP production via oxidative phosphorylation is able to keep pace with ATP hydrolysis.
White fibers predominate in quickly contracting muscles, such as the jumping muscles of frogs. These fibers are fast contracting because they have high myosin-ATPase activity. They fatigue quickly because ATP production via glycolysis does not keep pace with ATP breakdown. The proportion of red to white fibers in the leg muscles of humans appears to be determined genetically and is believed to account for individual differences between long distance and sprint runners.
During vigorous activity, blood supplying oxygen to and removing carbon dioxide from the muscles may be inadequate. Oxygen depletion of muscle promotes glycolysis and leads to the accumulation of lactic acid. On relaxation, oxygen reaches the muscle in adequate amounts and the lactic acid is removed by oxidation.
Following a lengthy period of muscle exertion one’s breathing rate may remain above normal for several minutes even though exercise is stopped. The extra oxygen consumed during this period is used to oxidize the accumulated lactic acid and is called the oxygen debt. Excess lactic acid is transported to the liver, where it is “recycled” first into glucose and then into glycogen.