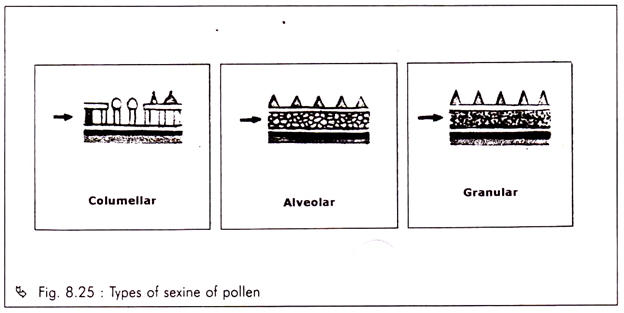
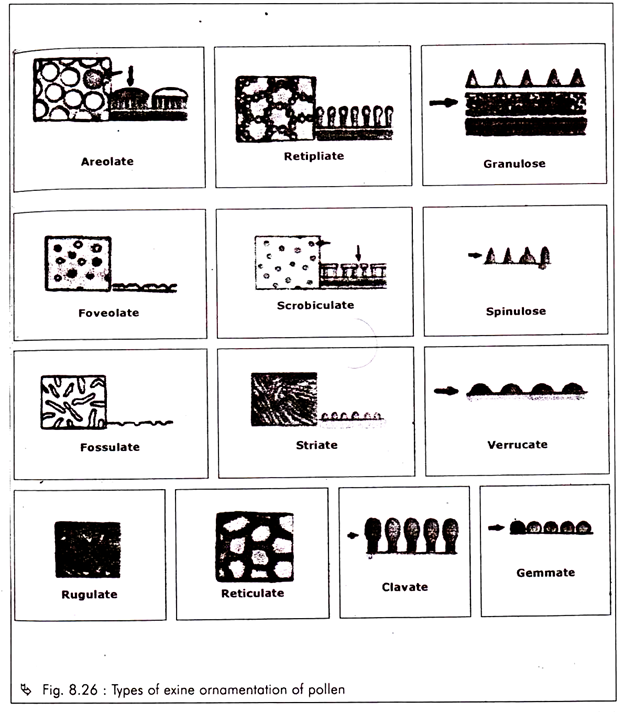
The special field of synecology which is concerned with the structure and classification of plant community is known as phytosociology.
The study of structure and composition of plant communities has been developed largely in Europe and Zurich-Montpether school of vegetational analysis led by J. Braun-Blanquet who has outlined several methods grouping them into phytosociology.
Two sets of characters, viz., analytical and synthetic are studied in a community at the same time.
Analytical Characters:
According to Hanson (1950) and Braun Blanquet (1932), analytical characteristics are those features of community which can be observed or measured directly in each stand. They include kinds and number of species, distribution of individuals, species vigour, form, number of individuals height of plants, area volume, growth rate and periodicity, etc. There are two different aspects of vegetational analysis-namely quantitative characters which can be measured more readily than the others, and qualitative characters (which are described and not measured).
Synthetic Characters:
Those aspects of community which are based on analytical characteristics and utilize data obtained in the analysis of a number of stands.
A. Qualitative Structures of Plant Community:
The qualitative structure and composition of plant community can be described on the basis of visual observations without any special sampling and measurement. In the qualitative Characteristics floristic enumeration (species content), stratification, aspection, sociability, interspecific associations, life-forms and biological spectrum, etc. are studied in the field.
1. Floristic composition or species content of community:
The study of species content in a community is of paramount importance. The species content of a community can be studied by periodic collection and identification of plant species for the whole year. This will show the tolerance of each species for different environmental conditions (Hanson, 1950).
2. Stratification and aspection:
The number of strata or layers in a community can be determined by general observation of the vegetation. If one periodically observes the flora for the whole year, changes in the appearance of vegetation may be apparent with the change in the season. This is known as aspection. For this phenology of species in relation to different seasons of the year is recorded.
The term phenology was first used by shelford (1929) to mark the events associated with seasonal, succession in natural communities. Later ecological society of America defined phenology as the science dealing with the appearance of certain events during the life cycle of an organism in different seasons of the year. Lieth (1970) defined phenology as the art of observing the life cycle or periodicity of plant and animals. Phenology, thus forms a part of community periodism. It is applied to those ecosystems where seasonal changes are not well marked such as desert cosystem.
On the basis of general observations of the vegetation a number of layers have been distinguished:
L1—ground stratum like mosses, thallophytes, lichens, etc.,
L2—herbaceous or ground flora,
L3—middle layer or shrubby layer, and
L4—top layer or canopy layer of trees.
In grasslands even two to three strata may be distinguished:
3. Life-forms:
On the basis of general appearance and growth, the species of community are grouped into different life-form classes. The chief criteria for recognizing life-form classes. On the basis of percentage values of different life-form classes, real nature of habitat and community can be understood.
4. Sociability:
In a plant community, the individuals of species are not evenly distributed. Individuals of some species grow widely spaced while those of some other species are found in clumps or mats. The space relationship of plants is referred to as sociability. Individuals of some species when growing in clumps are either very weak or they tend to disappear due to hard competition and as such they cannot form big populations.
Braun-Blanquet (1951) has recognized five sociability classes which accommodate different types of species:
Class 1. Shoots grow singly,
Class 2. Scattered groups or tufts of plants.
Class 3. Small scattered patches or cushions,
Class 4. Large patches or broken mats, and
Class 5. Very large mats of nearly pure population covering the entire area.
High degree of sociability is seen in those plants which produce large number of seeds with high germination percentage, show good survival of seedlings and mature plants and have many adaptive features. From gregariousness of species conclusions may often be drawn as to the nearness of approach to optimum conditions (Braun-Blanquet, 1932). This may explain the importance of the rough determinations of sociability values.
5. Interspecific associations:
When the plants belonging to two or more different species grow near one another they form a community. This type of association is known as interspecific association.
Interspecific association is possible if:
(i) The species can live in similar environment,
(ii) The species in question have similar geographical distribution,
(iii) The species belong to different life-forms (this reduces the competition), and
(iv) The plants of one species are related to the plants of other species. The relationship may be obligatory in one or both the directions.
Interspecific association can easily be observed in the field:
B. Quantitative analysis of Plant Community:
The structure of sociological order in any plant community cannot be studied by observing each and every individual of plant species growing in a habitat. It is rather impossible. Therefore, rough estimate of species content of a habitat is made by observing the plant species at different places or sample areas, in the habitat.
Several methods have been used by ecologists for this purpose which are as follows:
(1) Quadrat method,
(2) Transect method,
(3) Loop method, and
(4) Pointless or point method.
1. Quadrat Method of Sampling the Vegetation:
The quadrat is a square sample plot or unit for a detailed analysis of vegetation. It is actually the sample-plot method of Clements (1898). It may be a single sample plot or may be divided into several subplots. In vegetational analysis, quadrats of any size, shape, number and arrangement may be used. In the study of a forest community quadrats of one-fifth acre are established to include maximum number of trees, while for studying shrubs and grass covers usually the quadrats of smaller sizes are used (Fig. 6.4).
For grassland and low herbaceous community, the quadrats of one square metre size or 50 cm x 50 cm size or even 20 cm x 20 cm size may serve the purpose. The shape of quadrat is usually a square (Fig. 6.5 A and B) but rectangular or even circular ones are also used. In some cases rectangular sample plots often give the best results. The ratio of breadth and length in rectangular plots is generally 1: 2 or 1: 4 or 1: 8.
Kinds of Quadrats:
Quadrats are named according to the use.
These are of the following types:
(i) List quadrats:
When the organisms encountered in the sample plot are listed by their names, the quadrat is called list quadrat. It includes all the species botanically identified or otherwise. A series of list quadrats gives floristic analysis of the community. This is used for studying the frequency of different species.
(ii) Count quadrat or list-count quadrat:
When the species name and the number of individuals of each species found in the sample area are recorded, the sample plot is called count or list-count quadrat. This type of plot is usually used in forest survey work.
(iii) Cover quadrat:
When the actual or relative coverage is recorded usually as percentage of ground area covered or shaded by vegetation, the sample area is known as cover quadrat.
(iv) Chart quadrat:
Quadrats that are mapped to scales to show the location of individuals of species are called chart quadrats. Individuals plants are recorded on miniature quadrat on a graph paper often with the help of an instrument called pantograph. This is very tedious work but where long range studies of vegetational changes are being made, this method provides a big picture. So far as the distribution of quadrats in the study area is concerned, statistically reliable estimates are obtained by randomized plots.
This is done in the following way:
On the map of the area under study a series of horizontal and vertical grid lines are laid and then these lines are numbered. The numbers of horizontal and vertical lines are written separately on small square pieces of paper. The paper pieces bearing the numbers of grid lines are placed in two separate beakers (one for horizontal grid numbers and the other for vertical grid numbers) and paper-pieces in each beaker mixed. Then the pairs of numbers are drawn out from the beakers and the position of each plot is located by putting the point given by the paired numbers.
Species-area curve regards the selection of appropriate size of quadrat for sampling the vegetation, the quadrat selected should be of such small size as may cover the maximum number of species. There is a method for determining the minimal area of the sample plot. In this, sampling is done by using a geometric system of nested plots (Fig. 6.6). In plots of different sizes, number of species found in them are counted and recorded separately.
Then the numbers of species found in the plots of different sizes are plotted on vertical axis (O-Y axis) against sample plot sizes plotted on the horizontal axis (O—X axis). The resulting sigmoid curve will be obtained. This is called species-area curve (Fig. 6.7). Braun-Blanquet (1932), Oosting (1958), Misra and Puri (1954) and many other prominent ecologists of the world have used species-area curve for determining suitable area of the quadrat.
Such a curve is obtained because as the size of sample plot is increased the number of species increases in the initial stage but only up to certain plot size, and later the number of new species added declines and finally the curve tends to become horizontal. Thus there is little to be gained by increasing the plot size. The desirable minimal quadrat size is determined by locating the point on curve where line takes horizontal course and joining it to the horizontal axis will indicate the minimal size of small plot.
In a well stratified community, the study of different strata is done with the help of different sized quadrats. For the tree and shrub strata large sized quadrats are taken but for ground vegetation small sized quadrats are used. The minimal sizes of quadrat are determined by “species-area curve” method. In the same way, the minimum number of quadrats to be taken is also determined.
2. Transect Method:
A cross-section of an area used as a sample for recording, mapping or studying vegetation is called transect. It may be a strip, belt or a line across the area of study. The species occurring along these strips or lines are recorded. Because transect is continuous through the study area, it can be applied in studying the gradual and continuous changes in the vegetation along the line or strip with the change in environment. On the sloppy area, the transect is laid between two points at different altitudes.
The transects are of two types:
(i) Belt transect,
(ii) Line transect.
(i) Belt transect:
It may be established as follows:
(a) The total area of the site to be studied is divided by 5 or 10 to obtain the total number of sample areas.
(b) A series of belt transects of predetermined width and length are laid and the belts are divided into equal sized segments (Fig. 6.8). These segments are sometimes called quadrats, but they differ from true quadrats in that each of them represents one observation point.
Each segment within a belt is a part and the belt as a whole is one sampling unit.
(c) Names of species and number of individuals of each species in each unit are recorded.
The belt transect method is used to estimate abundance, frequency, and distribution of species in the community.
(ii) Line transect:
It is one dimensional transect. In this method, observation is taken on lines that are laid randomly or systematically over the study area (Fig. 6.8).
The procedure is as follows:
(a) A metric steel tape or steel chain is stretched between two stalks 33.5 metres or one chain apart.
(b) The line is considered to be a one centimeter wide belt extending along one side of the tape or chain.
(c) The observer moves along the lines and records plant species and the distance they cover along the line transect. For grasses, rosettes and dicot herbs, the distance covered is measured along the line at ground level. For shrubs and tall herbs, the shadow or foliage cover is measured.
(d) Twenty or thirty randomly placed lines under most conditions adequately sample the community.
The following information’s can be obtained by this method:
(i) The number of times each individual species appears along the transect.
(ii) The occurrence percentage for each species in relation to the total species.
(iii) The total linear distance in cm of each species along the line.
(iv) The total distance of intercept by all species per 30 m line.
3. The Loop Method:
This is a simple, accurate and quick method for sampling of only grassland and low herbaceous communities. It is used for determining community composition, species frequency and range condition. In this method equally spaced 100 small circles or loops located along a stretched line are used as observation points.
The procedure of loop method of sampling is as follows:
(a) A small wire loop of 2 cm diameter is made.
(b) A point is located at random in the community and from the point 33.5 m long steel tape is stretched out. The observation points are marked in each metre at 33 cm, 66 cm, and 100 cm mark. In this way 99 observation points are marked in 33 metre distance. Near the end of the tape at 33.33 metre mark an additional point is marked. This brings the total number of observation points to 100.
(c) At each observation point wire loop is dropped to the right side of the tape and species encountered in the circle is recorded.
In this method 20 to 30 transects under most conditions adequately sample the community. Since 100 observation points are used in each line, the sum-totals are read as percentage. By this method species contents and cover are easily computed.
4. Pointless or Point Method:
In this method of sampling observations are taken on the point in the study area where a nail or set of nails touch the ground on grid lines or at random places.
There are several point methods of sampling but here only the following two methods will be discussed:
(i) Point frame method.
(ii) Point centre method.
(i) Point frame method:
This method was introduced by Levy and Maiden (1933). It is done with the help of point frame. This consists of a scale like frame, supported by a pair of legs. The frame bears 10 equidistant holes having 60 cm long pointers or pins (Fig 6.9).
It is placed one after another at several observation points in the study area and the plant species that are hit by the pointed end of the Pointers or nails are recorded. Besides this, the number of times the species are hit and the total number of points taken are noted. From these values the quantitative structure of community is explained. This method is employed in the study of grassland and low herbaceous communities even on uneven ground.
(ii) Point Centre or Quarter method:
In this method of sampling four measurements are taken at each observation point. The observation points can be mechanically placed along a straight line or they can be located at random. Quarter method was first described by Cotton and Curtis (1956). In this method an easy instrument is used which consists of a brass needle or a nail fitted with rubber cork and compass on the top (Fig. 6.10).
The procedure of sampling is as follows:
(a) At each observation point the needle is fixed in the ground. This is central point.
(b) The working area is divided into four quarters or quadrats by visualizing two grid lines predetermined by the compass at right angles. Both the lines should cross each other at the central point.
(c) Now in each of the four quarters plant nearest to the central point is spotted and species recorded. The distance of each plant from the central point and also the basal diameter of the plant are measured.
(d) Tally at least 50 such points.
Quantitative Structure of Plant Community:
Coexistence and competition both are affected directly by the number of individuals in the community. Therefore, it is essential to know the quantitative structure of community. To characterize the community as a whole certain numerical constants called parameters are used. The total counts of individuals of each species, mean value of individuals of a species per plot, for example, are parameters.
Frequency, density, abundance, shadow and area coverage of species in the community, importance value index, total estimates, index of association, index of similarity, and fidelity of species give a clear picture of community structure in quantitative terms. The value of a parameter as estimated from the samples is the estimate which is hoped to be accurate or close to the real value.
1. Density:
The numerical strength of a species in relation to a definite unit space is called its density. The crude density refers to the number of individuals of a particular species per unit area, e.g., 2000 plants of Peristrophe species per acre will be the density of this species. Each organism occupies only the area that can adequately meet its requirements. Thus the density of an organism refers to the area available as living space. This would be ecological density.
Density of a species per unit area = Total number of individuals of a species in all the sample plots/Total No. of sample plots studied
Density of species in a field is determined by the method given in the following table:
In some cases, e.g., grasses and vegetatively propagated plants, the term individual creates difficulty. In such cases, each aerial tiller or shoot arising out from the soil is generally regarded as one individual.
The proportion of density of a species to that of stand as a whole is referred to as relative density.
The following formula is used for calculating relative density of a species:
Relative density of a species = Total no. of individuals of a species /Total no. of individuals of all species x 100
2. Frequency:
In the community, the individuals of all the species are not evenly distributed Individuals of some species are widely spaced while those of some other species are found in clumps or mats. The distribution patterns of individuals of different species indicate their reproductive capacity as well as their adaptability to the environment.
Frequency refers to the degree of dispersion in terms of percentage occurrence. In order to study the frequency of species in an area, the study area is sampled by any sampling method at several places in desired pattern or at random and only the names, not the numbers, of individual species encountered sample are listed.
The frequency of a species is determined with the help of the following formula:
Frequency = Total no. of quadrats in which the species occur/ Total no. of quadrats studied x 100
Suppose, species ‘A’ occurred in 4 quadrats out of total ten quadrats studied, the frequency of species A will be
4/10 x 100 = 40%
If the line or belt transect method is used for sampling then each line or belt is recognized as one quadrat for the purpose of frequency calculation.
If point frame method of sampling is used, the frequency is calculated by the following formula:
Frequency of a species = Total no. of hits the species secured x 100 / Total hits made
For calculating the frequency, the following pattern is adopted:
If the individuals of a species are evenly distributed over the area they may occur in all the sample plots and thus the frequency of species will be 100%. Poorly dispersed species will occur only in a few quadrats and their frequency will be low. This indicates that higher the frequency value of a species in the area the greater will be the uniformity in the spread. Raunkiaer recognized five frequency classes of plant species in the community on the basis of their frequency percentages.
These are as follows:
Class A—1 to 20% frequency
Class B—21 to 40% frequency
Class C—41 to 60 % frequency
Class D—61 to 80% frequency
Class E—81 to 100% frequency
Raunkiaer (1934) suggested that the number of species in frequency class A is greater than that of class B; B is greater than in class C, class C is greater, or equal or lesser than class D; and D is lesser than class E. A > B > C = D < E. This is also read as Raunkiaer’s ‘frequency law’. From the above frequency law it is apparent that the species with low frequency value are higher in number than the species with higher frequency value in most natural communities. The dispersion of species in relation to that of all the species is termed as relative frequency of a species.
Relative frequency is determined by the following formula:
Relative frequency of a species = Frequency of the species in stand x / Sum of the frequencies for all species in stand x 100
3. Abundance:
The estimated number of individuals of a species per unit area is referred to as abundance. To determine abundance, the sampling is done by quadrat or other methods at random at many places and the number of individuals of a species is added for all the quadrats studied.
The abundance is determined by the following formula:
Abundance of a species = Total number of individuals of the species in all quadrats /Total number of quadrats in which the species occurred
The abundance is usually expressed by assigning the species to one of the following abundance classes:
Abundance Classes:
Abundance refers actually to density of population in those quadrates in which a given species occurs. In low form of vegetation like grasslands abundance can be recorded by uprooting the plants.
4. Cover:
The cover implies the area covered or occupied by the leaves, stems and flowers, as viewed from the top. The coverage is studied at the canopy level and the basal region. In forest, where several strata are well marked, each layer of vegetation is considered separately for measuring the coverage. Basal cover is best expressed as the basal area, the ground actually covered by the crowns or by stems penetrating the soil. In forest the basal area is the cross section area of a tree measured at 4.5 feet above the ground (cross section area of a tree at breast height). It is estimated by point method of sampling (quarter method).
In grasslands, estimate of total spread of foliage has little meaning. Basal area in such cases refers to the coverage of ground one inch above the ground surface by stems and leaves. It is also called herbage cover (Fig. 6.11). The coverage can be measured by quadrat method, transect method and point method of sampling. Basal coverage of tree is measured at breast height.
Basal area of tree is calculated by the following formula:
Basal area per tree = Total basal area = dumber of trees. The area of coverage is used to express the dominance. The higher the coverage area the greater is the dominance. The average basal area is calculated out of average cross section areas of stems penetrating the soil. The average area of one stem multiplied by the density (no. of individuals per unit area) gives the basal cover per unit area.
Relative dominance (R.D.) is the proportion of the basal area of a species to the sum of the basal coverage of all the species in the area.
Relative dominance of the species = Total basal area of the species in all the quadrats/Total basal area of all the species in all the quadrats x 100
5. Total estimate:
Although abundance and coverage have their own importance in the community structure, yet they can be combined in a community description as total estimate. It is probably the best method for obtaining a complete general picture of a plant community.
Total estimate (abundance plus coverage) scale as suggested by Braun-Blanquet is as follows:
+ Individuals of a species very few; coverage very poor.
1. Individuals of a species in plenty; but coverage small.
2. Individuals numerous if small and a few if large; coverage 5% of the total area.
3. Individuals few or many; coverage 25 to 50% of the total area.
4. Individuals few or many; coverage 50 to 75% of the total area.
5. Plant species over 75-100% of the total area.
6. Association Index and Index of similarity:
The inter-specific association can be evaluated by association index and also by calculating the index of similarity. The index of similarity is utilized to compare two coexisting groups. Suppose, out of 100 quadrats studies species A is encountered in 90 quadrats and species A in association with another species B is found only in 40 quadrats, the association index of species A is calculated by dividing the total number of quadrats in which A occurred in association with B by the total number of quadrats in which species A is found (40/90 = 0.44).
Index of similarity is calculated as follows:
Suppose that in one group of coexisting species the number of plant species is 30 and in the other group the number of plant species is 20 and in first and second groups 15 species are common:
Index of similarity = 2 x no. of common species /Total number of species in both associations x 100
= 2 x 15 / 20 + 30 x 100 = 30 x 100 / 50 = 60
7. Importance value:
In any highly heterogeneous plant community, data of frequency, density, abundance, and cover of species do not yield total picture of ecological importance independently. The overall picture of ecological importance of a species in relation to the community structure can be obtained by adding the values of relative density, relative dominance, and relative frequency.
This total value out of 300 is called Importance Value Index (IVI) of the species. Once the importance values have been obtained for the species within the stands, the stands can then be grouped by their leading dominants according to the importance values and the groups are then placed in a logical order based on relationships of several predominant species. The dominants are arranged always in the order of decreasing importance values.
The importance of IVI was first pointed out by Curtis and Mcintosh (1951). The IVI, as pointed out earlier, gives complete picture of sociological character of a species in the community but it does not give the dimension of relative density, relative dominance and relative frequency. The individuals as well as combined aspects of the position of a species in the community structure can be shown with the help of phytographs.
In one type of phytograph a circle is made and then the circle is divided into four equal quarters by two diagonal lines lying at right angles to each other. Three radii from centre to circumference are divided into 100 segments and the fourth radius is divided into 300 parts (Fig. 6.12). On radius A is marked the value of relative frequency, on radius B is marked the value of relative density, on radius C is marked the value of relative dominance, and on D IVI on 0—300 scale. All these points on different radii are joined by lines. Thus, a phytograph illustrating the sociological characters and IVI of individual species is obtained.
Synthetic characters:
Synthetic characters describe the make-up of a community. The chief synthetic characters used and advocated by Braun-Blanquet (1932), Cain (1932) and Nichols (1930) are presence, Constance and fidelity.
Fidelity:
The term fidelity refers to the faithfulness of a species to its community. In the community, there are different types of species. Some plant species are confined to one particular community and they are called indicator species. Some can flourish in several communities. According to Hanson (1950), it is a measure of ecological amplitude. Pandeya (1960) observes that characteristic species with high fidelity value has low ecological amplitude.
Ecological amplitude of a species or its tolerance (Good, 1947) is the capacity of growing and reproducing within a certain range of environmental conditions. Fidelity of a species is expressed in relation to a particular community. A species may have high fidelity for one community and a low fidelity for another. Braun-Blanquet and Pavillard (1925), and Costing (1956) recognized the following classes of species on the basis of their fidelity.
Species of 3rd, 4th and 5th fidelity classes are called characteristic or key species of the community.
Presence:
It indicates the presence of a species in a stand. It is generally expressed in a scale of 1 to 5.
(i) Rare, which occur in 1% to 20% of stands examined
(ii) Seldom present, which occur in 21-40% of stands examined
(iii) Often present, which occur in 41-60% of the stands examined
(iv) Mostly present, which occur in 60 to 80% of stands examined
(v) Constantly present, which are present in 81 to 100% stands
Constance:
It is the degree of “presence” in a unit area (sample area) instead of the whole stand. It actually improves the method of presence study; otherwise there is no fundamental difference between presence and Constance. It is generally determined from frequency and the following 5 Constance classes have been recognized.
Con. 1—1 to 20% frequency
Con. 2—21 to 40% frequency
Con. 3—41 to 60% frequency
Con. 4—61 to 80% frequency
Con. 5—81 to 100% frequency