In this article we will discuss about the production of various enzymes.
Contents
1. Pectinases:
During the survey of crops for disease occurrence, it was observed that certain soft-rot causing bacteria produced pectolytic enzymes.The wilt disease induced by Fusarium species also produced pectinases as observed by Gothoskar 1955 and Waggoner and Dimond (1955).
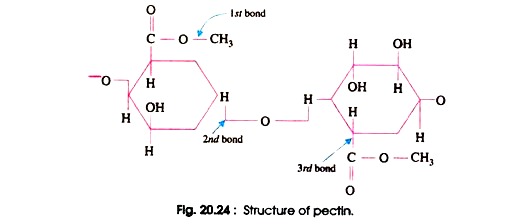
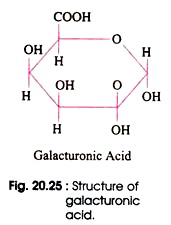
It was Scheffer and others who in the year 1950 contributed that Verticilluim species produces pectolytic enzymes that cause to the final plugging of xylem of infected plants. The production of appropriate pectolytic enzymes, however, may not be the only characteristics needed to make a wilt inducing fungus pathogenic to a particular species of plant. The pectic substances, maker of middle lamellae, are polymers of galacturonic acid (Fig. 20.24 and 20.25).
Two groups of pectolytic enzymes are produced by plant pathogens. One is pectin-esterase or pectin methyl esterase which breaks the ester bond i.e. 1st bond and removes CH3 group. The resulting products are pectic acid and methyl alcohol. The other enzyme is transeliminases which breaks 1, 4 glycosidic bond i.e. 2nd bond and removes H from bond 3rd, thereby un-saturating the ring between 4th and 5th C atoms.
(i) Production:
A number of commercial firms produce fungal pectinases using Aspergillus niger and Aspergillus wentii. Sometimes Rhizopus and Penicillium species are also used for pectinases production. The mycelium is developed on a medium containing pectin or a pectin like compound, a nitrogen source, such as yeast or malt extract, ammonia or peptone etc. and mineral salts.
The fermentation with A. niger runs for 60-80 h in fed-batch cultures at pH 3-4 at 37°C using 2% sucrose and 2% pectin. The purification is simple. Since the pectinase is present both in the cells (intracellular) as well as excreted to the medium (extracellular), the enzyme is recovered from both sources.
(ii) Harvest and Recovery:
At the time of harvest, the mycelium is dried and ground and its pectinase is extracted with water. It is then precipitated from this aqueous solution and from the culture broth by procedure as described for amylases extraction.
(iii) Uses:
Pectinases are used to clarify fruit juices and grapes must, for the maceration of vegetables and fruits besides extraction of olive oil. By treatment with pectinases for a period of 1 -2 h at 54°C or for a period of 6-8 h at 18°C, the yield of fruit juice during pressing is considerably increased. The commercial enzyme preparation contains at least two types of pectinases differing from each other by the extent to which they degrade pectin.
2. Invertase – (Saccharose or Sucrose):
This enzyme splits sucrose into glucose and fructose. It is widely distributed in nature. Saccharomyces fragilis, S. cerevisae and some other Saccharomyces sp. are the richest source of enzyme invertase. Invertase is produced in industries from baker’s or brewer’s yeast.
(i) Production:
Industrially, enzyme invertase is produced by special strains of yeast which grow on bottom. The medium contains sucrose, an ammonium salt, phosphate buffer and other minerals. The pH is adjusted to 4.5. Fermentation is carried out for about 8 h at 28-30°C.
(ii) Recovery:
The yeast cells are filtered off, compressed, plasmolysed and autolyzed. The invertase extracted may be dried or held in a sucrose syrup. The enzyme can also be purified by dialysis.
(iii) Uses:
Invertase is used in confectionery to make invert sugar for the preparation of ice creams, etc. Chocolate-coated candies are also prepared. This enzyme imparts de-crystallization of sugar in syrups on standing. Now-a-days, it has also been used in the manufacture of artificial honey.
3. Proteoses:
This group of enzymes catalyze the hydrolysis of the protein molecule. The enzyme is actually a mixture of proteinases and peptidases. These hydrolyze polypeptide fragements as amino acids.
(i) Microorganisms Involved:
Various bacteria, such as Bacillus, Pseudomonas, Clostridium, Proteus, Serratia, species and fungi, namely Aspergillus niger, A. oryzae, A.flavus, and Penicillium roquefortii are the sources of proteolytic enzymes.
(ii) Production:
A high yielding strain is selected and inoculated in special culture media containing 2-6% of carbohydrate, protein and mineral salt. It is incubated for 3-5 days at about 37°C with adequate aeration. The filtrate is concentrated, and the enzymes are used in this form from the culture is purified and absorbed onto some inert material such as saw dust.
It is always kept in mind that such strains are suitable for enzyme production which can give rise high yield of proteases and comparatively low yield of other enzymes. Many different media such as those containing wheat bran, soybean cake, alfalfa meal, are proved to be better for protease production.
(iii) Uses:
Bacterial proteases help in digestion of fish liver to release fish oil, to the tenderization of meat. Such enzymes are also used in beverage industries in clarification and maturing of malt beverages. On the other hand, fungal proteases are active in the production of soy sauce and other continental foods. They also remove protein haze from the beer and to hydrolyse the gelatinous protein material in fish waste.
Cheese manufacturing is a batch process and current practice is to use a milk-clotting enzyme in a soluble form. Immobilization of proteases for milk coagulation has received renewed interest and potential applications have recently been reported. Use of immobilized proteases would permit renneting of milk as described by Garg and Johri (1993).
4. Lipases (Glycerol Ester Hydrolase):
A large number of microorganisms are capable of using natural oils and fats as a carbon source for their growth. The enzyme responsible for the breakdown of the oils and fats prior to their digestion by microorganisms are extracellular lipases which catalyse the hydrolysis of triglycerides to free fatty acids, partial glycerides and glycerol.
(i) Microorganisms used in Fermentation:
Candida cylindracae, Candida rugosa, Aspergillus niger, Penicillium cyclopium, Humicola langinosa, Mucor javanicus, Rhizopus arrhizus, Geotrichum candidum have been reported for extracellular lipase production. Extraction of extracellular lipase from Pseudomonas aeruginosa has been studied by Jalger (1992).
(ii) Production:
Olive oil is being used as the substrate for the lipase enzyme. Stable emulsion of olive oil was prepared by vigorous mixing of the oil with a solution of an emulsion stabilizer Triton X-100 in 50 mM potassium phosphate buffer. The fermentation should be carried out at 29°C for 96 h supplied with 1% carbon source. The type of oil used proved to be important both lipase production and fungal growth.
(iii) Recovery:
The mycelia were filtered and the culture filtrate was subjected to lipase assay. Lipase determination can be done either by stirring method or by standing method.
(iv) Uses:
Miyoshi Oil and Fat Co. Japan is using Candida cylindracae lipase to hydrolyse oils for the production of soaps. Microbial liapases can also be used as catalysts for inter-esterification reactions. The application of lipolytic enzymes to flavour development in dairy products has been revived by Arnold etal (1975). Some Italian cheese are manufactured using lipases. Microbial lipases are also used in the formulation of clothes, washing products etc.
5. Celluloses:
The celluloses are synthesized by a large number of microorganisms, including fungi, bacteria, actinomycetes etc. Many bacteria utilize cellulose by cell bound enzymes, but fungi secrete cellulase into the growth medium. The extracellular celluloses secreted by Trichoderma ressei, T. koningii, Penicillum pinophiluns, Fusarium solani, Phanerochaete chrysosporium, etc. are among the best examples of celluloses.
Examples of aerobic cellulolytic bacteria are Cellulomonas sp., Cellubrio sp., Microbispora bispora, and Thennomonospora sp. Some of the anaerobic cellulolytic bacteria are Acetovibrio cellulolyticcus, Bacteroids cellulosovens, Clostridium thermocelluns, Ruminococcus albus.
(i) Production:
These fungi can be grown on basal medium given by Mendels and Sternberg (1976). This medium consists of all requirements for growth. The ingredients lit-1 are; potassium di-hydrogen phosphate (2g), ammonium sulphate (1.4g), magnesium sulphate (0.3g), calcium chloride (0.3g), urea (0.3g), peptone (1.0g), trace elements, ferrous sulphate (0.005g), manganese sulphate (0.0016g), zinc sulphate (0.0014g), cobalt chloride (0.002g) and pH 5.5.
The medium containing the enzyme-producing microorganisms (Trichoderma reesei) was then incubated at 35±1°C for 12 days. The mycelial mass is removed and the filtrate thus obtained acts as enzyme (crude).
Cellulase is a multi-enzyme complex, consisting of endo-β-glucanase, exo-β- glucanase and β- glucosidase. The endo-β-glucanase or carboxymethyl cellulase or Cx is assayed by determining the amount of reducing sugar released from the specific substrate carboxy methyl cellulose (CMC).
The mixture is boiled for 5 minutes at 100°C in a boiling water bath. It is cooled and optical density is recorded at 540 nm in a spectrophotometer. Exo-β-glucanase is measured by using Whatman No. 1 filter paper strip (50 mg) as substrate. The strip is dipped in 1.0 ml of citrate phosphate buffer (pH 5.5) and 0.5 ml culture filtrate.
This mixture was incubated at 50°C for 60 min. After incubation, 3.0 ml of DNS reagent is added and mixture is boiled for 5 min, cooled and diluted with distilled water. The optical density was recorded at 550 nm is spectrophotometer. The above enzymes give the approximate values of celluloses produced by any organism of industrial importance.
(ii) Recovery:
Cellulases can be recovered by following processes:
a. Process A:
By recycling of the insoluble residue as a source of cellulolytic activity.
b. Process B:
By adsorption of the soluble remaining cellulolytic activity onto fresh substrate.
c. Process C:
By combining both process A and process B.
In the process A, hydrolysate is centrifuged to get wet solid (bottom) phase. After washing, hydrolysis is carried on fresh substrate. The net production of sugar is determined which is further used to measure FPA/g (filter paper activity per gram) of substrate.
In the process B, recovery of soluble activity of the liquid supernatant phase of hydrolysate 1 is contacted at room temp. After centrifugation, the bottom phase is used to start hydrolysis 2. The net production of sugar is obtained which can be calculated in terms of FPU/g. On the other hand, maximum recovery may be obtained by combining both process A and B.
(iii) Uses:
Cellulases are being used in clarifying juices and in improvement of beer production. These are helpful in essential oils extraction from the plant tissues besides increasing the digestibility and nutritive value of plant products. Cellulases are also exploited in bioconversion of lignocellulosic waste into useful products such as glucose-fructose syrup, single cell protein cultivation and as fermentable sugars to alcohol.