Enzymes are essential for any chemical and physical changes within cells.
Thus, regulation of catalytic activity contributes in preserving homeostasis and understanding inborn-errors in metabolism.
Regulation of Enzyme action is accomplished by following ways:
(a) Compartmentalization:
Different enzymes with different tasks may be localized in specific sub-cellular compartments and locations. This ensures metabolic efficiency and simplifies regulation. For examples, chloroplasts contain photosynthetic enzymes, lysosomes contain hydrolytic enzymes, and mitochondria contain enzymes for TCA cycle, oxidative phosphorylation and energy metabolism.
(b) Covalent modification (Enzymatic inter-convertion):
Many enzymes are regulated by phosphorylation (addition of phosphate), dephosphorylation (removal of phosphate), adenylylation (addition of AMP) or other covalent modifications. Covalent modifications are themselves under enzymatic control and cause changes in tertiary structure of the enzyme that alter its catalytic activity. For example, protein kinases catalyze phosphorylation and the phosphorylated enzyme is active. Similarly, protein phosphatases catalyze dephosphorylation and the dephosphorylated enzyme is inactive.
(c) Partial proteolysis:
It is an irreversible covalent modification where inactive proenzymes or zymogens are activated by hydrolysis of one or more peptide bonds. For example, activation of protein digesting enzymes (proteases) only in digestive area avoids proteolysis of cellular constituents. Similarly blood clotting factors activate only at the site of cut which prevent internal clot (thrombus) formation.
(d) Control of Enzyme concentration:
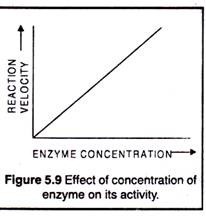
The concentration of a particular enzyme in a cell is determined by the rate of its synthesis and degradation. The rate of enzyme synthesis is regulated by induction and repression of gene, longevity of mRNA coding for enzyme. With only very few exceptions the rates of enzymatic reactions increase with increasing concentration of the enzyme (Fig. 5.9). Doubling the amount of enzyme doubles the reaction rate provided that pH, temperature and other conditions are not limiting.
With only very few exceptions the rates of enzymatic reactions increase with increasing concentration of the enzyme (Fig. 5.9). Doubling the amount of enzyme doubles the reaction rate provided that pH, temperature and other conditions are not limiting.
(e) Concentration of the Substrate:
The velocity of an enzymatic reaction usually increases with increase in the concentration of substrate up to certain maximum after which the relative amount acted upon per unit of time decreases with increase in the substrate concentration. The retarding effect may be due to very rapid accumulation of end products and decrease in water concentration (Fig. 5.11)
(f) Concentration of the End Product:
Enzymatic reactions, like all other chemical reactions, are subject to the laws of chemical equilibrium and mass action. Hence as the end products of the reaction accumulate the apparent rate of the reaction decreases (i.e. allosteric inhibition) sometimes, the enzyme itself combines with the end product thus reducing the rate further.
(g) Temperature:
The rate at which an enzymatic reaction proceeds is influenced by temperature. In general the initial velocity of enzymatic reaction is accelerated with increase in temperature until a certain optimum is attained. Above 40°C the destructive effect of temperature on most plant enzymes increases (Fig. 5.10).
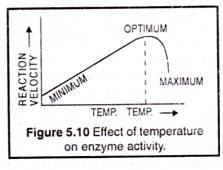 The optimum temperature for most enzymatic reactions has been found to be 40° to 50°C. This optimum temperature may fluctuate according to other conditions of the reactions, such as pH and concentration of the substrate.
The optimum temperature for most enzymatic reactions has been found to be 40° to 50°C. This optimum temperature may fluctuate according to other conditions of the reactions, such as pH and concentration of the substrate.
(h) pH of the Medium:
The activity of enzyme is greatly influenced by the hydrogen ions concentration of the medium in which the reaction occurs. The activity of enzyme appears to be maximum at a certain pH and to decrease rapidly on each side of this value. The optimum value varies from enzyme to enzyme and may range from as low as pH 1.5 to pH 10.
(i) Hydration:
The effect of increased hydration upon the enzyme activity of plant tissues is most easily demonstrated during the germination of seeds. As imbibition of water proceeds during germination the activity of the enzyme increases more or less progressively.
(j) Effect of the length of time:
As the reaction starts there is a linear relationship between amount of end product and the length of time the reaction has proceeded (Fig. 5.12), but eventually a stage comes after which there is no evidence of further enzyme activity even if some substrate is still present. This is usually considered due to inhibiting activity of the end product by changing the pH of the medium. Sometimes some other factor may also be involved.
(k) Activators:
Activators are some specific compounds which accelerate the rate of enzymatic reaction. The exact mechanism of their action is not understood. They may exert their influence by direct effect upon the enzyme or eliminating the effect of some substrate or factors which inhibit the operation of the enzyme. Some activators are general in the sense that they increase the activity of almost all enzymatic reactions e.g., salts of alkaline earth metals; others are highly specific for certain enzymes e.g., cobalt, manganese, nickel, magnesium and chlorine ions.
(l) Inhibitors:
Just as some compounds increase the rate of enzymatic reactions, there are others which inhibit the action of enzymes. These latter compounds are known as inhibitors.
They may be classified into two groups:
(i) Competitive and
(ii) non-competitive inhibitions.
(m) Feedback inhibition (=End product inhibition):
Feedback inhibition means the inhibition of an enzyme activity in a metabolic pathway by an end product of that pathway.
In a metabolic pathway (series of enzyme catalyzed reactions) when the biosynthesis of end products reaches a sufficiency high level then each end product act as an inhibitor or negative modulator and binds to the allosteric site of the first enzyme of the pathway to stop its catalytic activity. Thus, the first irreversible step called committed step of every metabolic pathway is subjected to the control of feedback inhibition.
(n) Radiant Energy:
Radiation of various wave lengths often has inhibitory or inactivating effects upon enzymes in vitro. The shorter wavelengths of the visible spectrum usually have more marked effects upon enzymatic activity than the large wave lengths. Very little is known regarding the effects of radiant energy upon enzymes as they exist in living cells.