Everything you need to know about enzymes. Some of the most frequently asked questions are as follows:-
Q.1. What is a cofactor in an enzyme?
Ans: Some enzymes require chemical groups other than their amino acid residues for activity. A cofactor may be either one or more inorganic ions like Fe2+, Mg2+, Mn2+, or Zn2+ or a complex organic or metal organic molecule called a coenzyme. Some enzymes require both a coenzyme and one or more metal ions for activity.
Q.2. What is a prosthetic group in an enzyme?
Ans: A coenzyme or metal ion which is covalently bound to an enzyme protein is called a prosthetic group. A complete catalytically active enzyme along with its coenzyme and or metal ions is known as holoenzyme.
Q.3. What is meant by Apo enzyme or Apo protein?
Ans: The protein part in a holoenzyme is called Apo enzyme or Apo protein.
Q.4. Define specificity of enzymes.
Ans: It is the ability of an enzyme or a receptor to discriminate among competing substrates or ligands. The ligand is a small molecule which binds specifically to a larger one, e.g. a hormone is a ligand for its specific protein receptor.
Q.5. What do you mean by catalytic power of biocatalyst’s or enzymes?
Ans: The rate enhancement produced by enzymes or biocatalyst’s are often in the range of 7 to 14 orders of magnitude.
Q.6. Where does the energy come from to provide a dramatic lowering of the activation energies for specific reactions?
Ans: The specific groups of an enzyme as specific amino acid side chains, metal ions and coenzymes lower the activation energy and thereby accelerate the reaction by providing a lower energy reaction path. In the formation of each weak interaction the enzyme substrate or ES complex is accompanied by a small release of free energy which gives a degree of stability to the interaction. The energy derived from enzyme – substrate interaction is known as binding energy.
The binding energy is a major source of free energy used by enzymes to lower the activation energies of reactions. The binding energy, in this way provides specificity as well as catalysis. Also, the weak interactions are optimized in the reaction transition state. The enzyme active sites are complementary not to the substrates per se rather to transition states of the reactions they catalyze. Lock and key model (Fig.40.1) is one of the most common explanations for interaction of substrate and enzyme.
Q.7. What is an active site in an enzyme?
Ans: The region of an enzyme surface which binds the substrate molecule and catalytically transforms it is called active site or catalytic site. The fatty acid synthase complex is known to have 7 different active sites. The fatty acid synthase system in Escherichia coli consists of seven separate polypeptides which are tightly associated in a single, organized complex. The proteins act together to catalyze the formation of fatty acids from acetyl – CoA and malonyl – CoA.
The intermediates in this whole process remain attached covalently to one of the two thiol group of the complex. One of the point of attachment is the – SH group of a Cys residue in one of the seven proteins (P – ketoacyl – ACP synthase) while the other is – SH groups of acyl carrier protein with which the acyl intermediates of fatty acid synthesis form a thioester.
Q.8. Give the requirements of active sites in enzymes.
Ans: The active site of an enzyme generally consists of a pocket on the enzyme surface lined with the amino acid side chains necessary to bind the substrate and catalyze its chemical transformation. For example in carboxypeptidase which leads to removal of carboxyl – terminal amino acid residues from its peptide substrates, comprises of a single chain of 307 amino acids. The two essential catalytic groups in the active site are furnished by Arg145 and Glu270.
Q.9. How do enzymes work?
Ans: Most biological molecules are quite stable in the neutral pH, mild-temperature, aqueous environment found inside cells. The reactions required to digest food, send nerve signals or contract muscle simply do not occurs at a useful rate in absence of catalysis.
The enzyme – Catalyzed reaction occurs within the confines of a pocket on the enzyme known as active site. The molecule which is bound by the active site and is acted upon by the enzyme is known as substrate. The enzyme substrate complex is central to the action of enzymes and it is the starting point for mathematical treatments defining the kinetic behaviour of enzyme – catalyzed reactions and for theoretical descriptions of enzyme mechanisms. A simple enzymatic reaction may be given as below:
Here E, S and P represent enzyme, substrate and the product.
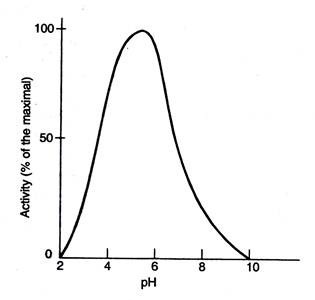
Q.10. When do enzymes typically exhibit maximum catalytic activity?
Ans: The enzymes typically show maximum catalytic activity at a characteristic pH known as optimum pH.
Fig 40.2. Optimum enzymatic activity of Lysozyme at pH 5.2
Q.11. How does uncontrolled diabetes of an individual turns fatal.
Ans: The pH of human blood plasma normally is 7.40. The pH regulating mechanisms fails when over production of metabolic acids causes’ acidosis. The pH of blood, so, may fall to 6.8 or below leading to irreparable cell damage and death. In other diseases the pH may rise to lethal levels. Therefore, biological control of pH of cells and body fluids is a matter of central importance in all aspects of metabolism and cellular activities.
Q.12. Can the pH changes are used to measure acetylcholine levels?
Ans: Yes, the concentration of acetylcholine a neurotransmitter can be determined from the pH changes which accompany its hydrolysis. When incubated with a catalytic amount of the enzyme acetylcholinestrase, acetylcholine is quantitatively converted into choline and acetic acid which dissociates to yield acetate and hydrogen ion.
Q.13. Define isozymes.
Ans: These are multiform of enzymes which catalyze the same reaction but differ from each other in their amino acid sequence, substrate affinity, Vmax and/or regulatory properties. These are also known as isoenzymes.
Q. 14. What is Cori cycle? Also discuss the isozymes (isoenzymes) associated with it.
Ans: The liver provides glucose to contracting skeletal muscle which derives ATP from the glycolytic conversion of glucose to lactate. The glucose is then synthesized from lactate by the liver. These conversions comprise the Cori cycle as shown in the figure below.
These conversions take place despite of differences in the catalytic properties of lactate dehydrogenase enzymes in skeletal muscle and liver. The lactate dehydrogenise is a tetramer of 35 – kDa subunits. There are two types of polypeptide chains referred to as M and H which can form 5 types of tetramers, which are: M4, M3H, M2H2, M1H3 and H4.
These species of enzymes are known as isozymes or isoenzymes. The M4 isozyme possesses a much higher affinity for pyruvate than the H, isozyme. The other isozymes have intermediate affinities. The principal isozyme in skeletal muscle and liver is M4 while the major one in the heart muscle is H4. Of course, these isozymes have been studied deeply but the reasons for the existence of their multiple forms yet remains unknown.
Q. 15. Which are two hallmarks of enzyme catalyzed reactions?
Ans: These are enormous activity and discriminating specificity. Crystallographic pictures of polypeptide chains of enzymes show that they are coiled in intricate shapes or conformations which apparently impart specificity and activity.
Q. 16. What is enzyme kinetics?
Ans: Much information may be obtained concerning the mechanism of enzyme – catalyzed reaction by kinetic studies, which means studies of reaction rates under various conditions, e.g. the reversible reaction. Where S is the substrate, P the product and E the enzyme. Under constant and suitable conditions of temperature and pH. The rate of reaction depends on the concentration of substrate [S], the concentration of product [P] and concentration of enzyme [E], The reaction velocity in the absence of enzyme is negligible. The velocity of overall reactions may be obtained by determining the changes in [S] or [P] with time.
In the study of kinetics of forward reaction alone one usually determines the velocity over a short time interval at the beginning of the reaction where P is still negligibly small. This has the additional advantage minimizing the effect of enzyme inactivation during the experiment.
Q.17. What is Michaelis – Menten equations?
Ans: A theory explaining the changes which take place in enzymatic reactions was proposed by Leonor Michaelis and Maud Menten in 1913. They postulated that substrate combined reversibly with enzyme to form an enzyme substrate complex [ES] which in turn is decomposed to yield the product and the free enzyme. The latter could then react with more substrate and the cycle is repeated.
Hence Michaelis – Menten equation can be defined as the equation that describes the hyperbolic dependence of the initial reaction velocity, Vo, on substrate concentration, [S], limited to early times in the course of the reaction. The biochemists Michaelis and Menten concerned themselves with the steady state rate and this type of analysis is called steady state kinetics.
Q.18. Define Michaelis – Menten constant (KJ.
Ans: It is the substrate concentration at which an enzyme – catalyzed reaction proceeds at one half its maximum velocity.
Q.19. Which characteristic of the enzymes makes them to follow Michaelis – Menten kinetics? Which enzymes are exceptions to it?
Ans: All enzymes which exhibit a hyperbolic dependence of Vo on [S] are said to follow Michaelis
– Menten kinetics. The regulatory enzymes and allosteric enzymes are exception to Michaelis
– Menten kinetics in many enzyme catalyzed reactions:
Q.20. What is Michaelis-Menten Kinetics?
Ans: A kinetic pattern in which the initial rate of an enzyme catalyzed reaction exhibits a hyperbolic dependence on substrate concentration.
Q.21. What is steady – state kinetics?
Ans: The enzyme while mixed first with a large excess of substrate there is an initial period called the pre-steady state during which the concentration of enzyme substrate complex increases or gets build up. Pre-steady state is usually too short to be easily observed.
The reaction soon achieves a steady state in which the ES and the concentration of any other intermediates is almost constant for some time. The measured V0 often reflects the steady state even though VQ is limited to early times in the course of the reaction.
Q.22. Name a best kinetic parameter used in comparison of catalytic efficiency.
Ans: The factor K cat /K m
Q.23. What is the usefulness of kinetic parameters Kcat and Km.
Ans: They are generally useful for the comparison and study of different enzymes to know whether their reaction mechanisms are simple or complex. Each enzyme possesses the optimum values of Kcat and Km which reflect the cellular environment.
Q.24. How can V mak and Km be determined or estimated?
Ans: They can be measured or determined by graphical methods.
Q.25. What are the multistep reactions? Discuss.
Ans: These reactions involve more than one step and are carried by multi enzyme complexes. The intermediates are channeled between glycolytic enzymes, which are a typical example. The enzymes of glycolysis usually described as soluble components of cytosol but there is growing evidence that within the cell exists the multi enzymes complexes.
There exists kinetic evidence for the channeling of 1, 3 biphosphoglycerate from glyceraldehyde – 3- phosphate dehydrogenase to phosphoglycerate kinase without entering solution is corroborated by physical evidence that the said two enzymes form stable complexes which are mono-covalent complexes.
Other examples are pyruvate dehydrogenase complex which requires 5 coenzymes; succinyl-CoA synthetase also called succinic thiokinase reaction; fatty acid synthase from bacteria and plants is a complex of 7 different polypeptides; construction of purine ring of insinuate (IMP) syntheses, synthesis of AMP and GMP from IMP, biosynthesis of pyrimidine nucleotides UTP (uridine – 5 – triphosphate) and CTP (cytidine – 5′ – triphosphate via orotidylate, and other biological oxidation reduction reactions.
Q.26. What do you mean by rate – limiting step?
Ans: A reaction consists of a number of steps out of which one or more steps are slowest (i.e., have lowest rate of reaction). These steps have highest activation energy. These steps are called rate limiting steps.Considering a simple case the rate limiting step is the highest energy point in the curve or diagrams that are often made for inter conversion of S and P, while S and P reaction (or S ![]() P) is catalyzed by an enzyme.
P) is catalyzed by an enzyme.
The ES and EP complexes are intermediates. These intermediates occupy valleys while reaction coordinate diagrams are drawn. In the citric acid cycle there are 3 strongly exergonic steps in the cycle which are catalyzed by enzymes citrate synthase, isocitrate dehydrogenase and a ketoglutarate dehydrogenase each of these enzymes can become a rate – limiting step under certain circumstances. The availability of substrates for citrate synthase (acetyl – CoA and oxaloacetate) varies with the metabolic circumstances sometimes limits the rate of citrate formation.
To conclude the rate-limiting step may be:
(1) Generally the step in an enzymatic reaction with the greatest activation energy or the transition state of highest free energy.
(2)It is the Slowest step in a metabolic pathway.
Q.27. What are suicide inhibitors?
Ans: The suicide inhibitors are a special class of irreversible inhibitors which are comparatively unreactive until they bind to the active site of enzyme. A suicide inhibitor is designed to carry out few chemical steps of the normal enzyme. But it instead of forming a normal product, the inhibitor is converted to a very reactive compound which combines irreversibly with the enzyme. Therefore, these have also been referred as mechanism based in-activators since they utilize the normal enzyme reaction mechanism to inactivate the enzyme. This sort of inhibitors find application in therapeutics by finding new pharmaceutical agents, a process known as rational drug design.
Q.28. Write a short note on allosterism?
Ans: Many enzymes have sites referred to as allosteric sites, which are quite different from the substrate binding sites. The legends that bind at the allosteric site are called allosteric effectors or modulators. Binding of an allosteric effector causes a conformational change of the enzyme, so that the affinity for the substrate or the ligand also changes. These effectors can bind reversibly and non-covalently to all allosteric sites and they affect the rate of reaction.
Positive (+) allosteric effectors increase the enzyme affinity for the substrate or other ligand. The reverse is true for negative (-) allosteric effector. This is a type of regulation known as allosterism and the enzyme regulated this way is referred to as an allosteric enzyme. The allosteric site to which the positive effector bind is referred to as an activator site; the negative effector binds at an inhibitory site.
Allosterism is an effective mechanism by which the enzymatic activities can be controlled to ensure that biological processes remain coordinated all times to meet the immediate metabolic requirement of a cell.
Q.29. What is an allosteric enzyme? Define.
Ans: It is a regulatory enzyme with catalytic activity modulated by non-covalent binding of a specific metabolite at a site other than the active site. The term allosteric has been coined from Greek allows = other, and stereos = solid or shape. Therefore, allosteric enzymes are those enzymes that possess “other shapes” or conformation induced by the binding of modulators. The activity of regulatory enzymes is modulated by means of various signal molecules which are generally small metabolites or cofactors.
Q.30. How are allosteric enzymes regulated?
Allosteric enzymes are regulated by non-covalent binding of modulators. The modulator or modulators are metabolites which when bound to the allosteric site of an enzyme alter its kinetic characteristics.
Q.31. Give the principles of allosteric regulation.
Ans: Allosteric enzymes do not follow Michaelis – Menten kinetics. The allosteric enzymes do not obey Michaelis – Menten display sigmoidal plots of the reaction velocity substrate concentration [S] rather than the hyperbolic plots predicted by the Michaels equation. Here, an important similarity lies that oxygen binding curve myoglobin is whereas that of haemoglobin is sigmoidal. Thus this example is analogous to enzymes two models have been proposed regarding the allosteric interactions.
These are:
1. The concerted model for allosteric interactions.
2. Sequential model for allosteric interactions.
Q.32. Which are the two models that explain the kinetic behaviour of allosteric enzymes? Explain.
Ans: These are as follows:
1. Concerted model or symmetry model:
This model was proposed by Jacques Monod, Jeffries Wyman and Jean – Pierre Changeux in 1965. According to this model allosteric enzymes can exist in two conformations, active and inactive. All subunits are in active form or all are in interactive form.
The assumption of this model is based on the thinking that both subunits must be in the same conformational state so that symmetry of the dimer is conserved. Every substrate molecule which binds increases the probability of a transition from the inactive to the active form.
2. Sequential model:
It was proposed by Koshland in 1966. There are two conformations but subunits can undergo the conformational change individually. According to this model there are more potential intermediate states in comparison to the symmetry model. However, a conformational change in one subunit makes a similar change in an adjacent subunit, enabling the binding of a second molecule more likely.