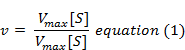This article throws light upon the six factors affecting the enzyme activity.
The six factors are: (1) Concentration of Enzyme (2) Concentration of Substrate (3) Effect of Temperature (4) Effect of pH (5) Effect of Product Concentration and (6) Effect of Activators.
The contact between the enzyme and substrate is the most essential pre-requisite for enzyme activity.
The important factors that influence the velocity of the enzyme reaction are discussed hereunder:
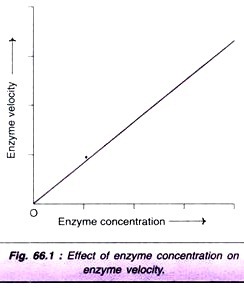
Factor # 1. Concentration of Enzyme:
As the concentration of the enzyme is increased, the velocity of the reaction proportionately increases (Fig. 66.1). In fact, this property of enzyme is made use in determining the activities of serum enzymes for diagnosis of diseases.
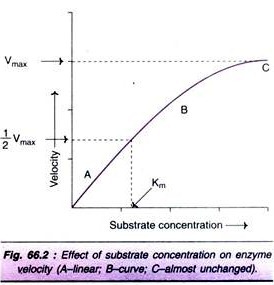
Factor # 2. Concentration of Substrate:
Increase in the substrate concentration gradually increases the velocity of enzyme reaction within the limited range of substrate levels. A rectangular hyperbola is obtained when velocity is plotted against the substrate concentration (Fig. 66.2). Three distinct phases of the reaction are observed in the graph.
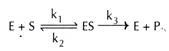
Enzyme kinetics and Km value:
The enzyme (E) and substrate (S) combine with each other to form an unstable enzyme-substrate complex (ES) for the formation of product (P).
Here k1, k2 and k3 represent the velocity constants for the respective reactions, as indicated by arrows.
Km, the Michaelis-Menten constant (or Brig’s and Haldane’s constant), is given by the formula
The following equation is obtained after suitable algebraic manipulation.
where v = Measured velocity,
Vmax = Maximum velocity,
S = Substrate concentration,
Km = Michaelis-Menten constant.
Km or the Michaelis-Menten constant is defined as the substrate concentration (expressed in moles/lit) to produce half-maximum velocity in an enzyme catalysed reaction. It indicates that half of the enzyme molecules (i.e. 50%) are bound with the substrate molecules when the substrate concentration equals the Km value.
Km value is a constant and a characteristic feature of a given enzyme. It is a representative for measuring the strength of ES complex. A low Km value indicates a strong affinity between enzyme and substrate, whereas a high Km value reflects a weak affinity between them. For majority of enzymes, the Km values are in the range of 10-5 to 10-2 moles.
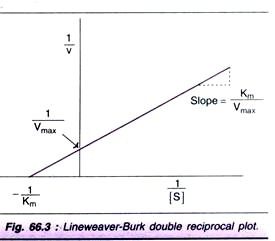
Line weaver-Burk double reciprocal plot:
For the determination of Km value, the substrate saturation curve (Fig. 66.2) is not very accurate since Vmax is approached asymptotically. By taking the reciprocals of the equation (1), a straight line graphic representation is obtained.
The Line weaver-Burk plot is shown in Fig. 66.3. It is much easier to calculate the Km from the intercept on x-axis which is -(1/Km). Further, the double reciprocal plot is useful in understanding the effect of various inhibitions.
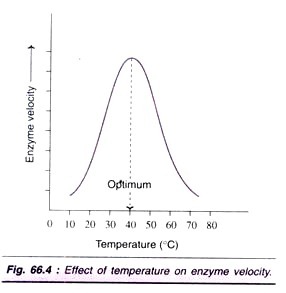
Factor # 3. Effect of Temperature:
Velocity of an enzyme reaction increases with increase in temperature up to a maximum and then declines. A bell-shaped curve is usually observed (Fig. 66.4).
The optimum temperature for most of the enzymes is between 40°C-45°C. However, a few enzymes (e.g. venom phosphokinases, muscle adenylate kinase) are active even at 100°C. In general, when the enzymes are exposed to a temperature above 50°C, denaturation leading to derangement in the native (tertiary) structure of the protein and active site are seen. Majority of the enzymes become inactive at higher temperature (above 70°C).
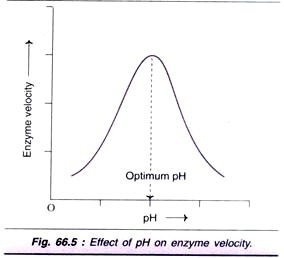
Factor # 4. Effect of pH:
Increase in the hydrogen ion concentration (pH) considerably influences the enzyme activity and a bell-shaped curve is normally obtained (Fig. 66.5). Each enzyme has an optimum pH at which the velocity is maximum.
Most of the enzymes of higher organisms show optimum activity around neutral pH (6-8). There are, however, many exceptions like pepsin (1-2), acid phosphatase (4-5) and alkaline phosphatase (10-11) for optimum pH.
Factor # 5. Effect of Product Concentration:
The accumulation of reaction products generally decreases the enzyme velocity. For certain’ enzymes, the products combine with the active site of enzyme and form a loose complex and, thus, inhibit the enzyme activity. In the living system, this type of inhibition is generally prevented by a quick removal of products formed.
Factor # 6. Effect of Activators:
Some of the enzymes require certain inorganic metallic cations like Mg2+, Mn2+, Zn2+, Ca2+, Co2+, Cu2+, Na+, K+ etc. for their optimum activity. Rarely, anions are also needed for enzyme activity e.g. chloride ion (CI–) for amylase.