Read this article to learn about Recovery Methods of Bio-products!
A preliminary assessment of the techniques developed up to 1968 was obtained from a summary of detailed isolation procedures published in the Journal of Biological Chemistry in 1968.
This survey was carried out by Dunnill et al. and is given in Table 10.1.
In the left hand column are the main isolation steps that were used, followed by a percentage of published reports in which those isolation steps occurred more than once. On the right hand side are the most common methods or materials for each isolation step and the percentage of occasions when they were applied at a given step.
From the survey the most common set of five steps following extraction is nucleic acid removal, protein precipitation, ion-exchange, gel filtration and adsorption. The average yield is between 58 and 90%. The average overall yield is 18% and the average overall purification of cells need five steps.
To date many more techniques have been developed on a laboratory scale and could be considered for large scale application. It must be emphasized that the technology indicated in this is based on published data and as a result is some-what limited as most information relating to large scale equipment and processes is still being treated as proprietary information by suppliers. Table 10.2 summarizes the main classes of separation techniques available today.
The degree of separation attained by these different methods depends upon the contribution made by the forces F1 and F2 to their net difference ∆F. Thus, in chromatography for instance, the F1 forces are mainly hydrodynamic and are very similar for all molecules. The high selectivity of the process depends to a large extent on the types of forces involved in separation. Table 10.2 shows the different conventional separation processes available for the separation of components of a mixture along with the types of forces (F1 & F2) involved in each of the processes.
General Factors Related to Large Scale Purification Procedures:
The main criteria for the scale up of any process are technical viability and economic feasibility. The main unit operations for the purification of enzymes are basically the same for both the laboratory and large scale operations. However, certain problems which are inherent in large scale operations are often not noticeable in the smaller scale. Some of these problems are foaming, temperature control, high shear stresses and extended process time.
Aeration of solutions during transfer, mixing or centrifugation may generate considerable foam and unit operations such as pH adjustment or the addition of reagents may become difficult. Large centrifuges, for instance, generate a considerable amount of heat and temperature control is maintained by the circulation of a coolant in a jacketed tank and by the use of special equipment.
Control of process time is a most frequent problem. Certain steps require a longer period to complete on a large scale than in the laboratory, and cause enzyme instability, which may not be apparent in the laboratory, becomes evident and a problem.
Once a particular step is recognized as being time- sensitive, the preparation is either divided into several batches or a continuous process is employed at that point. Ideally, the ultimate minimization of process time could be achieved with the development and utilization of a completely continuous process.
The options available for scale up are:
(i) Large scale batch equipment.
(ii) Large scale semi-continuous or batch-continuous operations.
(iii) Large scale continuous operations.
There can be no rigid set of rules as to the best system, as in many cases the level of development of the technology and the related economics often dictate the final decision. It would be appropriate at this stage to briefly examine the use of batch and continuous systems for enzyme purification.
It is much simpler to initiate a batch process than a continuous one. However, once in operation, the continuous process can operate automatically whereas the batch process requires constant attention, especially during time sensitive stages.
The development of a truly continuous process requires the co-ordination of every stage so that desired conditions prevail. This inevitably involves the use of automatic controls. It must be borne in mind those most analytical procedures developed at the laboratory scale for enzymes involve the degradation of at least part of the sample. Adaptation of such procedures for on stream analysis and process control would be very difficult.
Selection of Purification Methods:
The criteria for selecting purification methods are largely dependent on the final use intended for the enzyme and the purity required.
The important considerations are:
(i) Proposed use of the enzyme, e.g. L-asparaginase must be void of pyrogens and toxins; detergent enzymes must satisfy FDA standards with a microbial count of less than 50,000.
(ii) Cost of production.
(iii) Enzyme stability: This is particularly important as it dictates the entire course of purification. It must be emphasized that the stability conditions found with crude extracts will only be valid during the early stages of purification as the apparent chemical properties of the enzyme may change during the course of isolation, owing to progressive removal of substances which complex or interact with it.
(iv) Relative location of the enzyme: Apart from intracellular or extracellular, the enzyme may be part of a complex or agglomerate.
(v) Nature of the organism:
The final yield of a particular enzyme from a process may be greatly influenced by the choice of organism and the growth conditions. Several techniques such as strain selection; modification of genetic composition; induction; etc., are available for improving enzyme yields.
The following basic data should also be known about the enzyme:
1. Solubility:
a. In water or dilute solutions
b. In polar solvents such as methanol, ethanol, acetone
c. In weakly polar solvents such as chloroform and hydrocarbons
2. Stability:
a. pH range
b. Temperature effect
c. Effects of organic solvents
3. Stability in the solid state:
a. Temperature effect
b. Effect of moisture content
4. Physical properties:
a. Dialysability through membranes of different pore sizes
b. Adsorption on solids
c. Migration in an electric field as a function of pH
d. Sedimentation in a centrifuge
5. Chemical properties:
a. Stability towards proteolytic enzymes
b. Stability after acetylation under mild conditions
c. Stability to esterification under mild conditions
The stability data 2.a-2.c, are the most important preliminary investigations. Most of the enzymes of biochemical importance are stable over a limited pH range and prolonged separation processes may require to be carried out at reduced temperatures.
Generally, the successive stages should be based on the different properties of the enzyme. Thus, if purification has been achieved by making use of the anionic properties of the enzyme further purification is more likely to be successful by making use of some other property of the enzyme such as its cationic properties. Fig. 10.1 summarises the general purification steps which may be used. A technique which would reduce the final cost of the enzyme is to design the purification stages so as to purify several enzymes from one source. This is practical, however, only when use exists for many enzymes from the same source.
Scale up of Specific Purification Procedures:
1. Cell disruption:
(i) General:
The techniques for cell breakage are summarized in Fig. 10.2.
Table 10.3 gives a synopsis of the relative effectiveness of some of the methods of cell disruption.
(ii) Theoretical Background:
The non-mechanical models are limited in application to large scale protein release. The approach to data has to use data, collected on laboratory devices for the adaptation of industrial machines already used extensively in the chemical process field. Sonication, too, is limited with regards to the high capital investment involved when related to separation efficiency. Such a system may only be feasible when a battery of units in parallel are considered. Equipment design must be accounted for adequate exposure and mixing of the fluid/cell mixture.
The machines available for mechanical disruption are:
(i) Colloid mill
(ii) Ultrasonic probe
(iii) Homogenizer
(iv) High speed ball mill
(v) Extreme pressure pump
Only (iii) to (v) will be considered as the rest are limited to pilot scale. Dunnill et al. carried out detailed studies on protein release on cell disruption using a Manton-Gaulin APV homogenizer (The APV Co., Ltd., Crawley, Surrey, U.K.).
They obtained the following correlation:
Where R – Amount of soluble protein released after N cycles through the homogenizer
Rm – Maximum release of soluble protein
K – kPx [fn(T)] – A temperature dependent 1st-order rate constant
P – Operating pressure
k, x – Constants for a given microorganism grown under particular conditions, x for yeast was 2.9.
They found no evidence of protein denaturation during multiple passes. Increase in cell disruption with pressure was supported by Brookman, who obtained 100% cell breakage at a pressure of 1750 kgf/cm2. He produced evidence to indicate that the main mechanism responsible for the cell breakage was pressure. He also observed a linear increase of temperature with pressure (1.5°C/70 kgf/cm2) at a flow rate of 59 l/hr, and a 60% w/v yeast suspension. Several factors influence the performance of homogenizers of different size.
The main factors concerning the use of these machines in large scale operations are:
(a) The correlation given as in equation 10.1 is applicable up to pressures of 1200 kgf/cm2, and is useful in determining the required number of passes for a particular degree of disruption.
(b) The increase in temperature at increased flow rates and pressure must be well known. There must be adequate cooling between passes if protein denaturation due to heat is to be avoided.
(c) Commercial homogenizers working with high pressures are available with nominal capacities up to 9000 l/hr and there is a possibility that these could be adapted for use.
(d) Power efficiencies (% disruption/kw), at different operating pressures must be carefully analysed before the decision is made to use machines of higher capacities and operating pressures.
(e) This system could be operated on a continuous basis as indicated in Fig. 10.3.
Mogren et al. obtained data for the use of a high speed ball mill for cell disruption. The machine used had a capacity of 180 l/hr (Model KD5 Dyno-Mill, W.A. Eachofen, Easel, Switzerland). 85% cell disruption and an electricity requirement of 0.3 kwH/kg of dry yeast were observed. Cooling was provided by an outer jacket to the grinding chamber.
The main factors of interest here are:
(a) Cooling facilities would be inadequate as the equipment size is increased. Although different cooling media could be used, heat exchange surface area would be limiting. Provision must be made for the inclusion of an external heat exchanger in system so as to minimize the risk of denaturation.
(b) This system may produce high enough shear forces on a large scale to cause denaturation.
(c) Rotary mills tend to produce size segregation which could affect mixing, and even more- so at larger diameters. This would reduce the overall breakage efficiency.
(d) Power efficiencies must be compared with other classes of machines as well as other sizes of the same design. The efficiencies would be higher than for a homogenizer.
(e) Adhesion of the suspension to the walls of the containing vessel will be more significant on a large scale operation. The design may become too intricate if baffle/scrapers have to be used.
(f) Recovery of solid particles used to enhance grinding would require additional unit operations which could add significant costs to a large scale operation.
Brookman et al. investigated the use of a high pressure pump (700-3100 kgf/cm2) for pumping the cell suspension through a needle valve 90% cell breakage was obtained at flow rates of 2 l/hr (60% W/V yeast suspension).
The main considerations here are:
(a) Increase in temperature will be due to the reduction in volume at high pressures in the needle valve. Cooling could be provided by jacketing the line from the pump up to end including the valve. Heat exchange surface area would be the limiting factor on large scale operation. A large pump coupled to a manifold leading to a battery of valves may be a possible solution.
(b) Capital investment may be higher for this system.
(c) Power efficiency will be lower.
(d) Maintenance costs due to the repair and replacement of valves will be significant.
2. Enzyme purification:
(i) General Background:
The main methods of enzyme precipitation are:
(a) Isoelectric point:
At the isoelectric point there are no electrostatic repulsion forces between neighbouring enzyme molecules that coalesce and precipitate. When the pH of the enzyme mixture is adjusted to the isoelectric pH of one of its components, much or all of those components will precipitate leaving behind in solution enzymes with pH values above or below that pH. The precipitated isoelectric protein remains in its native conformation and can be re-dissolved in a medium having an appropriate pH and salt concentration.
(b) Salting out:
At low concentration, certain salts increase the solubility of most enzymes. The ability of neutral salts to influence the solubility of enzymes is a function of their ionic strength, a measure of both the concentration and number of electric charges on the cations and anions contributed by the salt. As the ionic strength is increased the solubility of the enzyme begins to decrease. At a sufficiently high concentration the enzyme may be almost completely precipitated from the solution-an effect called salting out.
(c) Solvent:
The reduction of the effective dielectric constant of a solution of a polar solute in a polar solvent by the addition of a weakly polar solvent will frequently result in the precipitation of the solute. When applied to enzymes however, caution must be exercised, since a medium of low dielectric constant increases electrostatic interactions between ionic groups and reduces dispersion interactions between nonpolar groups. Denaturation may result.
(d) Thermal differential denaturation:
If the extraneous enzymes denature more rapidly than the desired enzyme, they precipitate, leaving the desired enzyme in solution.
(ii) Discussion:
Precipitation is basically a simple first stage tool for the purification of enzymes from solution and allows further fractionation to be carried out with smaller volumes leading to decreased processing time. Important to large scale operation are the physical characteristics of the final suspension.
The following general problems must be considered:
(i) The rate of addition of the precipitating agent affects the particle size.
(ii) The rate of mixing (turbulence created) affects the particle size and also leads to heat generation in the system. A high degree of turbulence would ensure homogeneity of the mixture but may lead to protein denaturation.
(iii) Heat removal from the system must be efficient (especially so when organic solvents are used).
(iv) The high viscosity of crude protein extracts compounds poses the problems of adequate mixing and efficient heat removal.
(v) A method of agitation which may be suitable on the laboratory scale may not be on the large scale.
Methods (i)—(iii) could be carried out in similar equipment, although (iii) is applicable in special cases due to the problem of denaturation mentioned above. Only (ii) and (iv) will be discussed here.
On salting out Dixon and Webb13, investigated the mechanism involved and put forward the following equation relating the solubility of a protein, s(gm// of soln.) with the ionic strength:
where β and Ks are constants and Γ/2 the ionic strength in moles/l of solution. Hence, if an enzyme is present at a concentration s1 and requires an ionic strength of salt I1 for precipitation to occur, once the specific constant Ks is known, then the ionic strength I2 required for precipitation of the enzyme at concentration s2, could be calculated from the following relation:
The work of Foster et al., at a later date indicated that equation (10.2) was useful for the purposes of estimation but they emphasized the need to study these systems in the regions where the equation was not valid and salting out occurs at lower ammonium sulphate concentrations as the enzyme concentration increases.
The following factors are particularly relevant to large scale operation:
(i) The temperature must be carefully controlled. Variation of temperature introduces a difficulty in the use of percent saturation as a measure of salt concentration since the solubility of the salt will vary. In addition, since the effects of a change of temperature are different with different enzymes the order of precipitation of a given series of enzymes as the salt concentration is increased, may be quite different at different temperatures. In large scale operation temperature control is not instantaneous and adequate cooling capacity must be provided.
(ii) Protein concentration is an important variable. Excess dilution must be avoided since dilute solutions require considerably more salt to achieve the same degree of precipitation.
(iii) Control of pH is difficult e.g. ammonium sulphate is slightly acidic, is not a buffer and there is always a slight loss of ammonia. Special pH control instruments would be required for a large system.
(iv) In the design of large systems using high concentrations of salts, allowance must be made for the fact that there are considerable volumetric changes when amounts of salt of the required order are dissolved in water.
(v) Repeated fractionation using the same conditions is of limited value and higher separating efficiencies are obtained by repeating the fractionation under different conditions.
(vi) The rate of addition of salt may affect the equilibrium state.
(vii) Residence time required for the process would be important in equipment selection.
The following types of equipment are suggested for large scale operation:
(a) CSTR:
The degree of mixing is the important consideration here.
A mixing parameter is defined by the following equation:
(b) Column operation:
The intrinsic problem here is the difficulty of obtaining equilibrium between liquid and solid phases sufficiently quickly to permit efficient cascade operation. The continuous extraction of a precipitated material with a gradient of increasing solvent properties is possible, e.g. a gradient of salt precipitant such as ammonium sulphate could be established in a molecular sieve column of suitable porosity so that the salt concentration increases downwards towards the outlet.
(c) Cyclone mixers:
A simple system which could probably be used in this type of application is a modified forced circulation crystallizer as indicated in Fig. 10.4.
This system would, offer the following advantages:
(i) Adequate mixing
(ii) Adequate residence time
(iii) Continuous flow
(iv) Good temperature control.
(v) Low cost preliminary separation after precipitation.
On thermal denaturation following equations were developed for scale up based on the assumptions that thermal denaturation is a first order reaction.
If the protein concentration of a given species remaining un-denatured at time 9 is x, and if x0 is the initial concentration, then
The activation energy for denaturation for a particular enzyme may be determined from the rate of loss of activity at two different temperatures:
A mean value for H for several proteins is 82000 cal/mole.
The equipment which could be used for this system is as follows:
(i) A battery of stirred jacketed vessels using steam, ethylene glycol, etc., as heating medium in the outer jacket: These vessels are standard equipment used in most food industries and selection could be based on standard heat exchange principles.
(ii) Shell and tube heat exchangers: These too are standard equipment.
The overall heat transfer coefficients for these systems could be determined by simple heat balance experiments. The main limitation to the use of shell and tube heat exchangers is the possible denaturation of the required enzyme due to shear forces at the turbulent Reynolds numbers required for efficient heat transfer.
There would obviously be an optimum balance. In addition there may be a maintenance cost due to the plugging of the heat exchanger although this may be partially solved by passing the enzyme suspension on the shell side of the exchanger. The use of this system is, of course, limited to particular enzymes e.g. glucose isomerase.
Chromatography:
1. General concepts:
Chromatography is defined as the uniform percolation of a fluid through a column of more or less finely divided substance, which selectively retards, by whatever means, certain components of the fluid.
The following types of systems are of interest for the large scale purification of enzymes:
(i) Adsorption:
The solute is retained on an adsorbent surface as a result of dipole interactions, Van der Waals or London dispersion forces, and hydrogen bonding between solute and adsorbent. The principal environmental factor influencing the interaction between a solute and adsorbent is the solute-solvent interaction. Both forces are similar and energy changes are of the same magnitude. The extent of adsorption is influenced by a small change-in one of them.
(ii) Partition:
The basic mechanism is the partition of the solutes between two immiscible liquid phases. Selection of solvent systems which will provide suitable distribution ratios (D) or partition coefficients (α) is essential for good separation (a = D (Vm / Vs) where Vm and Vs are the volume of mobile and stationary phases respectively).
(iii) Ion-exchange:
This involves the stoichiometric exchange of ions across the solid- liquid interface. Exchange can involve either anion or cation. It is based on the principle that different enzymes have different ionic charges at different pH values and are attracted or repelled accordingly.
(iv) Affinity:
This method is based on the attachment of one of the reactants to an inert, non-adsorbent and insoluble supporting matrix and the use of this matrix for the selective removal of the other reactant. After thorough washing to remove retained contaminants, the retained reactant could then be removed by elution under suitable conditions.
This method is particularly appealing for industrial application since:
a. Purification in a single step may be as much as 4000.
b. Complete biological purity may be achieved.
c. Can be operated at very high loading.
d. It may be directly applicable to clarified cell extract.
The disadvantages are:
a. It must be developed afresh for each product or group of products.
b. The bound component causing specific retention is often costly.
c. The solid chromatographic phase must be highly porous and hydrophillic so that its mechanical strength tends to be poor.
d. Elution conditions tend to be harsh or expensive. Special eluent is required.
(v) Molecular sieve:
This is based upon separation due to the different sizes of different enzyme molecules.
Of particular interest in large scale chromatography are the concepts of zonal separation and gradient solution. Zonal separation is ideally the resolution of the components of a mixture into discrete zones which are separated by regions of solute free solvent. The components become separated into discrete zones on account of diffusing affinities for the stationary phase.
When gradient elution is used, the eluting power of the solvent is gradually and continuously increased by an automatically programmed change in some property such as solvent composition, ionic strength or pH. In this way, the solutes which are firmly retained by the stationary phase under the initial eluent conditions can be eluted as sharp separation zones.
The following equations are useful for initial scale-up of the column:
Ve=V0 + aVs (10.9)
D= 1 – R/R (10.10)
Where D – Distribution ratio, ratio of mass of solute in the stationary phase to the mass of solute in the mobile phase (D = ts/tm).
Ve– Elution volume (Ve = Fte)
V0 – Column void volume: total mobile phase volume in the column.
a – Partition coefficient, ratio of solute concentrations in stationary and mobile phases
Vs – Total stationary phase volume in the column.
R – Solute relative parameter, ratio of the linear rate of movement of a solute zone maximum to the linear rate of movement of the solvent (dimensionless).
te – Total time taken for a solute molecule to traverse the column.
ts, tm – Time spent by solute in stationary and mobile phases
F – Volumetric flow rate in column.
2. Different chromatography methods:
(i) Column Chromatography:
The chromatographic methods identified in the previous section could all be applied to a large scale column and the important scale up parameters are the same.
The major causes of increased zone dispersion are:
(i) Longitudinal diffusion of solute in both phases
(ii) Lack of attainment of equilibrium due to resistance to mass transfer
(iii) Dispersion phenomena arising from non-uniform liquid flow through a cross-section of column
The dispersion effects depend on the following:
(i) Flow rate
(ii) Stationary phase particle size
(iii) Column dimensions
The most important parameters which could be manipulated to optimise resolution are:
(i) Column geometry
(ii) Column packing
(iii) Particle size
(iv) Flow rate
(v) Solvent properties
(vi) Temperature
(vii) Inlet flow distribution
Scale up must, therefore, take into consideration the following:
(A) Column geometry:
Considering the zone spreading characteristic equation in the following form:
It is significant to note that is directly proportioned to L1/2. It indicates that while the differential migration of different components in the mixture is proportional to L the overall resolution is only increasing by a factor of L1/2. An approach to scale-up is to use constant L/D (length to diameter) ratio and Reynolds number (NRe = DV r/m where V = superficial liquid velocity; m, r = viscosity and density of eluting fluid respectively), creating a situation of dynamic similarity between columns.
Since porosity and packing are the same in each case, then the term DV is of particular significance. Charm et al. used this approach for scale-up of molecular sieve chromatography but it should be applicable to columns using other methods, with slight modifications.
They concluded that if these two dimensionless groups were kept constant, then the quality of the elution pattern will be the same in each column. They also found that for a molecular sieve system the following relationship is applicable:
log ((L /D) / DV) = A (∑ max / minute) + B (10.12)
Where A and B are constants and ∑ max/minute is a measure of the degree of separation achieved in the column (this is obtained by summing the maximum values of the second and third peaks of the elution pattern and dividing the total by the minimum value between them).
(B) Column packing:
The column must be packed to avoid segregation of the particles according to size, both longitudinally and laterally. This could lead to channeling in the bed.
(C) Particle size:
Ideally there should be uniformity of particle size. Uneven reduction of particle sizes will occur due to attrition. Use of small particles leads to increased pressure drop and decreased flow rate. Size need to be optimized considering surface area and hydraulic problems.
(D) Flow rate:
High flow rates are required in most situations but care must be exercised to always operate below the “flooding velocity”. High flow rates lead to bed compaction and increased pressure drop. The two methods of introducing the feed into the column are by a positive pressure at the top of the column or by a section at the bottom of the column.
The latter method is expensive and may result in the formation of gas bubbles in the section of the packed bed near the outlet. Although excess pressure applied to the solvent inlet stream may produce a similar effect on the upper section of the packing, this is less harmful as it alters the local column volume before the zones of the individual components have been separated. With the advent of mechanically stronger particles high pressure chromatography could be used in some cases. High pressure systems would require the use of reciprocating pumps and surge tanks or pressure transducers to reduce pulsations.
(E) Inlet distributor:
Distribution becomes more problematic as the column diameter is increased. Charm et. al. investigated the design of inlet distributors for large scale columns. Open cones of small diverging angles were found to be poor inlets. Multiple screens gave good velocity distribution but poor dispersion performance.
Inlet cones packed from the larger end with 70-80% of their volume gave adequate distribution and exhibited excellent dispersion properties. A system which was not investigated (according to the literature) but which may be suitable is the use of an annulus with open cone nozzles so located that their range of spray overlaps.
(F) Solvent properties:
Low viscosity solvents reduce the pressure drop across the bed and enhance distribution.
(G) Temperature:
Increase in temperature reduces the partition coefficient and causes a decrease in resolution with reduced enzyme stability.
(H) Mass transfer:
The two basic mechanisms are longitudinal and lateral. These processes serve to establish local equilibrium within the column and affect the resolution. Hamilton et al suggested the following equation for the height of the theoretical plate in ion-exchange chromatography (a modification of this equation should be applicable to other systems):
Where H – Height of the theoretical plate
λ – Constant
k – Mass transfer coefficient
Ds – Diffusion coefficient of the solute and the stationary phase
F – Linear flow rate
D – Distribution ratio
dp – Exchange particle diameter
The role of lateral diffusion was investigated by Giddings, who suggested various coupling mechanisms and emphasized the importance of analysing each system individually.
(i) Collection attractions:
This would require a sophisticated control system if the column is operated continuously. The best approach would be the use of a battery of columns operating as a batch continuous system so that flow could be continuous downstream of the chromatographic section of the plant.
(ii) Continuous Gel Chromatography:
A diagram of such a system is given in Fig. 10.5. In this system, the solute mixture is fed continuously while the individual solutes are recovered continuously from suitable ports.
An annular bed of the stationary phase is contained in the space between two concentric cylinders A and B, and packed in it to the level C. After passage through the “column” the eluent is removed at a series of equidistant drip points E, arranged at the bottom of the annulus.
This system was investigated by Fox et al. who applied it successfully in the purification of skim milk proteins. This system is limited for large scale operation; however, it is believed that at high rotational speeds separation may be hindered by the resulting centrifugal forces.
3. Fluidized bed chromatography:
The use of fluidized bed system was considered for counter current ion-exchange chromatography. Although this was eliminated in preference to a packed bed, it is believed that there is potential in the use of fluidized beds for chromatographic separations. This is so as such systems offer a high degree of mixing and expose greater surface area for mass transfer. The main limitation may be denaturation due to high shear forces.
Filtration:
1. Membrane ultrafiltration (UF):
(I) General Background:
This is the hydraulic pressure mediated separation of solutions into their individual components by passage through synthetic membranes. The process depicted in Fig. 10.6 utilizes specially constituted polymeric films as molecular screens which discriminate between solute and solvent molecules on the basis of size, shape or chemical structure.
UF offers the following advantages for industrial application:
(i) The process is athermal and permits the removal of water up to 90% at ambient and other selected temperatures, avoiding thermal and oxidative degradation of the product. However in such cases solute diffusion coefficient is temperature dependent.
(ii) Since there is no phase change, it avoids collapse of gels, breaking of emulsions and mechanical damage associated with freezing.
(iii) No solvents or other precipitating agents are required for the concentrating process. The basic mass transfer mechanism involved with tight membranes (those capable of retaining solutes whose molecular diameters are about 10 Ᾰ or less) is diffusive transport (Fickian).
In these membranes both solute and solvent migrate by molecular diffusion within the polymer; driven by concentration gradients established in the membrane by the applied pressure difference. Loose membranes (those retaining particles larger than 10 Ᾰ) appear to function as molecular screens. Solvent moves through the membrane micro-pores in essentially viscous flow while solute molecules are carried convectively with solvent, but only in those pores whose dimensions are large enough to accommodate them.
(II) Influential Factors:
The following factors are important for scale up:
(A) Concentration polarization:
This is the major hindrance to the use of UF on a large scale. The criteria for local steady state mass transfer of solute require that the rate of convective transport of solute towards the membrane surface be equal to the rate of transport (by diffusive and convective mechanisms) of solute away from the surface.
This condition can only be satisfied if the solute concentration in the layer of solute adjacent to the membrane surface is higher than in the bulk of the solution within the channel. This is the concentration polarization which is common to every mass transfer process occurring across a semi-permeable membrane.
The consequence of polarization is the formation of a boundary layer of substantially more concentrated solution (relative to mid-channel concentration) adjacent to the membrane. As the fluid moves down the channel and becomes more and more solvent depleted, the bulk and boundary layer solute concentrations increase and the thickness of the solute enriched boundary layer increases. This process is depicted in Fig. 10.7 and 10.8.
The following adverse effects of concentration polarization are important:
(i) If the membrane is not completely retentive for solute, the ultra-filtrate will contain a substantially higher concentration of solute that would be predicted from the bulk excellent dispersion properties for a system which has solute concentration in the upstream solution. Hence the membrane’s apparent rejection efficiency will be substantially reduced relative to its rejection efficiency.
(ii) If the solution is sufficiently concentrated and the solute of relatively low molecular weight, the high solute osmotic pressure in the boundary layer will reduce the effective pressure for UF, thereby reducing the UF flux.
(iii) If the solution contains a high molecular weight solute, accumulation at the membrane surface produces a large hydraulic flow resistance.
This system has been analyzed by several worker, and the following equations could be applied for scale up of channel flow:
It should be noted that these equations do not consider molecular or eddy diffusion in the boundary layer.
(B) Salt concentration:
In the preparation of enzymes, the aqueous suspension often contains a combination of salts. Membrane manufacturers often report the performance of their products in terms of filtration rates for distilled water. This method of reporting the performance index is almost meaningless for biological solutions.
The UF rate decreases with increasing molar concentration. The membrane does not usually retain the salts but due to their ionic nature some intermolecular association occurs and the UF rate is ultimately decreased. This problem would lead to serious discrepancies in equipment capacity on a large scale.
(C) Effect of pressure and temperature on the UF rate:
The effect of pressure and temperature are important for several reasons. If the rate can be increased by a proportional increase in pressure, this would allow one to use a smaller UF unit to accomplish a given task. Thus the gain in the filtration rate could lead to a reduction in the equipment cost.
Thermal instability of enzymes will dictate the upper operating temperature so that it is desirable to know the effects of temperature changes on the membrane behaviour. Generally, there is an increase in filtration rate with pressure. However, this is not a linear increase in rate and the linearity decreases with increasing temperature and pressure. This suggests that membranes may become deformed with increasing pressure and so careful selection must be made.
(D) Effectiveness of shear in clearing membrane surface:
The greatest shear rate does not necessarily produce the greatest filtration rate. When shear strength of the resistance cake is the dominating factor limiting filtration, then high shear rate and the associating high shear stress at the membrane surface should be the effective means for reducing the resistance.
High shear rates tend to inactivate the enzyme. Table 10.4 gives an idea of the effect of different systems on the enzyme at different shear rates. However, as turbulence and vibration are effective in improving the flow rate, an optimum balance between effective flow rate and inactivation must be obtained.
(E) Simplified filtration model for scale-up:
This model is applicable to UF and other types of filtration systems.
The flow of filtration through a filtration medium is given by:
Where V – Volume of filtrate
A – Area of membrane
P – Pressure drop across membrane
K1 – Constant characteristic for a given material deposited
Rm – Resistance of membrane
t – Time
2. Equipment:
UF could be used in anyone of the following systems on a large scale operation:
(i) Batch
(ii) “Feed and bleed” operation
(iii) Multi-stage continuous
Multistage continuous operation could be used for both purification and concentration or as a continuous counter current system as indicated in Fig. 10.9 and 10.10. This is most suitable for large scale operation. In recent years protein transmission capacity of UF membranes has been reported.
3. Other filtration techniques:
The following methods are available for the filtration of cell debris and precipitated protein:
(i) Rotary vacuum
(ii) Filter press (Leaf and Plate and Frame)
These systems could use filter aids such as charcoal to assist in the filtration process. A technique which is applicable to the removal of solids at this stage of the purification process is cross flow filtration. This basically involves the retention of un-dissolved (particulate) material by the filtration barrier with tangential suspension flow.
Analysis of cross flow filtration is similar to that for ultra-filtration. The equipment which could be used here is available for various scales and application is a question of adaptation rather than actual design. The simplified model put forwarded in earlier section is also applicable here.
Electrical Field:
The methods which used electrical field in molecular separation are the following:
(a) Isoelectric focusing:
This is limited to a laboratory scale primarily due to the problem of pH gradient instability on a large scale.
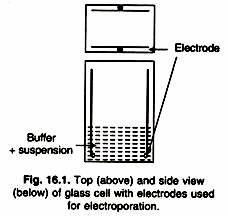
(b) Electrophoresis:
This too is limited to a laboratory scale. The problems here are the high generation of heat due to the electric current and the complicated equipment which will be required for separation of a mixture of enzymes on a large scale.
Centrifugation:
The use of centrifuges is somewhat limited on a large scale because:
(i) Refrigeration costs are quite high.
(ii) As the size is increased the cost of the equipment is very high due to the cost of fabrication.
The only system which may be feasible would be a battery of continuous centrifuges connected to the same outlet manifold.
The following equation is applicable to scaleup:
Where Q – Allowable flow rate
Y – Length of bowl
w – Angular rotational speed
r1, r0 – Outside and inside radii of bowl
Dp – Particle diameter
μ – Viscosity of suspending liquid
pL‘ pp – Density of liquid and particle respectively
Fs – Correction factor for particle interactions
Counter Current Distribution:
This method involves the separation of the components of the mixture by treatment with a solvent in which one or more of the solvents are preferably soluble.
The following problems must be considered in the scale up of this method:
(i) Enzymes are unstable when subjected to mechanical agitation in a two-phase liquid system at room temperature. This may be due to the tendency for denaturation to occur at the very large liquid-liquid interface.
(ii) Controlled low temperatures may be required and the solvents used must be suitable for low temperature operation.
(iii) Foaming would have to be minimised.
The equipment required for this technique is similar to that used in liquid-liquid extraction, and scale up would involve similar mass transfer equations.
Foam Separation:
1. General:
This is a method of separation the components of a solution by utilizing differences in surface activity. The principle underlying this process is that enzymes partition themselves between the bulk and the surface region of an aqueous system. However, when two or more enzymes are present in the same solution, then the concentration ratio in the foam will be different from that of the bulk. This is as a result of different adsorptions of the components present.
The following factors are important for scale up here:
(i) Enzyme Concentration:
The enrichment ratio increases with decreasing concentration of protein in the bulk solution. In a batch process, as the concentration of the enzyme preparation is increased, a monolayer forms resulting in decreased surface tension and the presence of a surface excess. As the foam separation continues, the concentration of the enzyme in the bulk solution decreases until there is insufficient protein for stable cell formation. It is important to operate at a low concentration to get a high enrichment ratio but a concentration high enough to produce foaming.
(ii) Flow Rate:
Enrichment ratio increases with decreasing gas flow rate, down to a limiting flow rate where the gas bubbles arrive too infrequently at the top of the solution for foam to form. The bubbles must have adequate residence time for solute adsorption.
(iii) Foam Height:
The higher the foam column is allowed to rise before foam is removed to give a foam, the greater will be the enrichment due to drainage and/or coalescence of the surface excess layers comprising the foam.
(iv) Environmental Factors:
Enzymes are most susceptible to foaming at pH values near the iso-electric point. Temperature is not significant within the normal ambient range.
(v) Denaturation:
The risk of denaturation is reduced by using an inert gas to produce foam, by avoiding mechanical agitation and by using a once through process rather than a recycle one. The use of an inert gas may affect process economics significantly.
Table 10.5 gives an idea of purification of catalase and amylase by this method.
2. Equipment:
The different systems that could be used for large scale operation are given in Fig. 10.11.
The following equations are applicable for steady state flow in a continuous flow stripper: Tall column
Where CF, Cw, CQ – Concentration of enzyme to be separated in the feed stream and foamate.
Γw – Surface excess in equilibrium with Cw
S – Surface to volume ratio for a bubble (for a sphere S = 6/d)
G, F, Q – Volumetric flow rates of gas, feed and bottoms
D – Volumetric flow rate at which net foamate is removed.
Scale up of this equipment could produce channelling or significant deviation from plug flow of the foam. This could be minimized by using a low L/D ratio or lowering the liquid and gas flow rates.