Read this article to learn about the organotypic model. The organotypic model consists of three approaches for the original structural and functional interactive relationships of the organ.
The three approaches are: (1) Organ Cultures (2) Histotypic Cultures and (3) Organotypic Cultures.
The cell cultures are widely used in the laboratories world over for various purposes. In vitro studies with isolated cells are useful for understanding of many cell functions such as transcription, translation, cell proliferation, respiration and glycolysis. Thus for the study of biology and many functions, the cells grown in conventional and monolayer cultures may be adequate.
However, for the study of integrated cellular functions or organ functions, isolated cells will be not be of much use, as explained below:
Cellular Interactions in Organ Functions:
There occurs interaction among various cells in vivo, resulting in a cascade of events. These cellular interactions (mostly due to hormonal stimulation) are very important for the expression of their functions, as indicated by the following examples.
a. Hormonal stimulation of fibroblasts is responsible for the release of surfactant by the lung alveolar cells.
b. Androgen binding to stromal cells stimulates prostate epithelium.
Besides hormones, nutritional factors and xenobiotic also exert stimulatory effects on the cells to function in a coordinated fashion.
Organotypic Models:
The cellular interactions that occur in the in vivo system are not possible with isolated cells. The recent developments in the organ and histotypic cultures focus to create in vitro models comparable (as far as possible in biology and functions) to the in vivo systems. The purpose of this organotypic models is to retain the original structural and functional interactive relationships of the organ.
There are three broad approaches in this direction:
1. Organ cultures:
The whole organs or small fragments of the organs that retain the special and intrinsic properties are used in culture.
2. Histotypic cultures:
The cell lines grown in three dimensional matrix to high density represent histotypic cultures.
3. Organotypic cultures:
In this case, the cells from different lineages are put together in the desired ratio and spatial relationships to create a component of an organ in the laboratory.
1. Organ Cultures:
The use of organ cultures (organs or their representative fragments) with reference to structural integrity, nutrient and gas exchange, growth and differentiation, along with the advantages and limitations is briefly described.
Structural Integrity:
As already stated, the isolated cells are individual, while in the organ culture, the cells are integrated as a single unit. The cell to cell association, and interactions found in the native tissues or organs are retained to a large extent. As the structural integrity of the original tissue is preserved, the associated cells can exchange signals through cell adhesion or communications.
Nutrient and Gas Exchange:
There is no vascular system in the organ culture. This limits the nutrient supply and gas exchanges of the cells. This happens despite the adequate care taken in the laboratory for the rapid diffusion of nutrients and gases by placing the organ cultures at the interface between the liquid and gaseous phases.
As a consequence, some degree of necrosis at the central part of the organ may occur. Some workers prefer to use high O2 concentration (sometimes even pure O2) in the organ cultures. Exposure of cells to high O2 content is associated with the risk of O2 induced toxicity e.g. nutrient metabolite exchange is severely affected.
Growth and Differentiation:
In general, the organ cultures do not grow except some amount of proliferation that may occur on the outer cell layers.
Advantages of Organ Cultures:
i. Provide a direct means of studying the behaviour of an integrated tissue in the laboratory.
ii. Understanding of biochemical and molecular functions of an organ/tissue becomes easy.
Limitations of Organ Cultures:
i. Organ cultures cannot be propagated, hence for each experiment there is a need for a fresh organ from a donor.
ii. Variations are high and reproducibility is low.
iii. Difficult to prepare, besides being expensive.
Techniques of Organ Culture:
The most important requirement of organ or tissue culture is to place them at such a location so that optimal nutrient and gas exchanges occur.
This is mostly achieved by keeping the tissue at gas- limited interface of the following supports:
i. Semisolid gel of agar.
ii. Clotted plasma.
iii. Micro-porous filter.
iv. Lens paper.
v. Strip of Perspex or Plexiglas.
In recent years, filter-well inserts are in use to attain the natural geometry of tissues more easily.
Procedure for Organ Culture:
The basic technique of organ culture consists of the following stages:
1. Dissection and collection of the organ tissue.
2. Reduce the size of the tissue as desired, preferably to less than I mm in thickness.
3. Place tissue on a support at the gas medium interface.
4. Incubate in a humid CO2 incubator.
5. Change the medium (M199 or CMRL 1066) as frequently as desired.
6. The organ culture can be analysed by histology, autoradiography and immunochemistry.
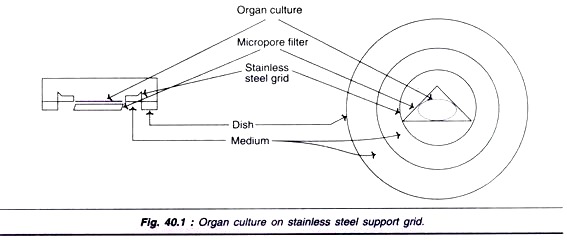
Organ Culture on Stainless Steel Support Grid:
Small fragments of tissue can be cultured on a filter laid on top of a stainless steel grid (Fig. 40.1).
Organ Culture on Filter-well Inserts:
Filter-well inserts have become very popular for organ cultures. This is mainly because the cellular interaction, stratification and polarization are better in these culture systems. Further, the recombination of cells to form tissue — like densities, and access to medium and gas exchange are better.
The four different types of filter wells for growing tissues in the form of cell layers are depicted in Fig. 40.2.
i. Growth of cell layer on top of filter (Fig. 40.2A).
ii. Growth of cell layers on matrix (collagen or matrigel) on top of filter (Fig. 40.2B).
iii. Cell layers grown on the interactive cell layers placed on the underside of filter (Fig. 40.2C).
iv. Cell layer grown on the matrix with interactive cell layer on the underside of the filter (Fig. 40.2D).
Filter well-inserts with different materials (ceramic, collagen, and nitrocellulose) are now commercially available for use in culture laboratories.
Filter-well inserts have been successfully used to develop functionally integrated thyroid epithelium, stratified epidermis, intestinal epithelium and renal (kidney) epithelium.
2. Histotypic Cultures:
Growth and propagation of cell lines in three- dimensional matrix to high cell density represent histotypic cultures. The advantage with this culture system is that dispersed monolayer cultures can be used to regenerate tissue-like structures. The commonly used techniques in histotypic cultures use gel and sponge hollow fibers and spheroids.
Gel and Sponge Technique:
The cells (normal or tumor) in culture can penetrate gels (collagen) or sponges (gelatin) which provides a matrix for morphogenesis of primitive cells. This approach has been used for the development of mammary epithelium, and some tubular and glandular structures.
Hollow Fibers Technique:
In recent years, perfusion chambers with a bed of plastic capillary fibers have been developed. The advantage of using hollow fibers in histotypic cultures is that nutrient and gas exchange is more efficient. As the cells attached to capillary fibers grow, there occurs an increase in cell density to form tissue-like structures.
Many workers claim that the behaviour of high-density cells formed on hollow fibers is comparable to their in vivo behaviour. For instance, choriocarcinoma cells grown in hollow fiber cultures release more chorionic gonadotrophin than in a conventional monolayer. Hollow fiber culture techniques are regarded as ideal systems for the industrial production of several biologically important compounds. Work is progressing in this direction.
Three Dimensional Cultures:
Spheroids in Histotypic Culture:
Spheroids represent the clusters of cells usually formed by the re-association of dissociated cultured cells. It is known for some years that the dissociated embryonic cells reassemble to form a specialized structure. The basic principle of using spheroids in histotypic culture is that the cells in heterotypic or bomotypic aggregates are capable of sorting out themselves into groups to form tissue-like architecture. The major drawback of spheroids is the limitation in the diffusion and exchange of nutrients and gases.
Multicellular Tumor Spheroids (MCTS):
Multicellular tumor spheroids provide an in vitro proliferating model for studies on tumor cells. The three dimensional structure of MCTS allows the experimental studies related to drug therapy, penetration of drugs, resistance to radiation etc.
Further, MCTS have also been used to study several biological processes:
i. Regulation of cell proliferation and differentiation.
ii. Immune responses.
iii. Cell death.
iv. Cell invasion.
v. Gene therapy.
The main advantage of three dimensional cell cultures (in the form of MCTS) is that they provide a well-defined geometry of cells planar or spherical which is directly related to the structure and function. It is now well accepted that the MCTS behave like the initial avascular stages of solid tumors in vivo. However, beyond a critical size (≥ 500 mm), most of the MCTS develop necrosis (death of cells) at the centre surrounded by viable cells. A diagrammatic representation of MCTS in comparison with tumor is depicted in Fig. 40.3.
Technique of MCTS production:
Single-cell suspension obtained from trypsinized monolayer cells or disaggregated tumor is inoculated into the medium in magnetic stirrer flasks or roller tubes. As the incubation is carried out for about 3-5 days, aggregates of cells representing spheroids are formed. It is observed that spheroid formation is more efficient under static conditions on stationary and non-adhesive surfaces. For this reason, agar/agarose-coated culture dishes to which cells do not adhere are frequently used to initiate spheroid formation.
Once the spheroids are formed, they are transferred to 24 well plates for analysis. Spheroid growth is quantified by measuring their diameters regularly. This can be done by using a microscope eyepiece micrometer or an image analysis scanner. Good growth of spheroids is observed when grown in wells.
Transfectant mosaic spheroids:
It is now possible to produce spheroids from cells that have been transfected with different genes. Mosaic spheroids are formed by mixing transfected and non-transfected spheroids in the desired proportion.
MCTS co-cultures:
MCTS can be produced from heterogenous cells also, forming MCTS co- cultures. This is comparable to heterologous spheroids (in short heterospheroids) consisting of tumor cells in combination with host cells.
Some of the MCTS co-cultures are listed:
i. MCTS and immune cells.
ii. MCTS and fibroblasts.
iii. MCTS and endothelial cells.
Heterospheroids with heterotypic cell interaction serve as good models for studying several in vivo processes e.g. inflammation. MCTS co-cultures are very useful in tissue modelling and tissue engineering, the details of which are given later.
Applications of Spheroids or MCTS:
Spheroids have a wide range of applications. Some of the important ones are listed:
i. Serve as models for a vascular tumor growth.
ii. For the study of gene expression in a three dimensional configuration of cells.
iii. To determine the effect cytotoxic drugs, antibodies, radio nucleotides used for therapeutic purposes.
iv. To study certain disease processes e.g. rheumatoid arthritis.
v. For the development of gene therapies for several diseases e.g. cancer.
vi. To evaluate radiation effects on target tissues.
vii. For the development of tissues and tissue models.
3. Organotypic Cultures:
Organotypic culture basically involves the combination of cells from different lineages in a determined ratio to create a component of an organ. With the advances in the organotypic culture techniques, it is now possible to develop certain tissues or tissue models.
i. Skin equivalents have been created by co-culturing dermis with epidermis with interviewing layers of collagen.
ii. Models for prostate and breast.
iii. Models for control of growth and differentiation of lung.