Read this article to learn about the scope and applications of biotechnology.
The applications of biotechnology includes plant tissue culture, production of transgenic in animal and plants, applications in medicine as tools and therapeutics, creation of new enzymes and their immobilization for industrial use, development of monoclonal antibodies and control of pollutions, etc.
1. Introduction:
Biotechnology is defined as the ‘application of scientific and engineering principles to the processing of material by biological agents to provide goods and services’. The Spinks Report (1980) defined biotechnology as ‘the application of biological organisms, systems or processes to the manufacturing and service industries’. United States Congress’s Office of Technology Assessment defined biotechnology as ‘any technique that used living organisms to make or modify a product, to improve plants or animals or to develop microorganisms for specific uses’.
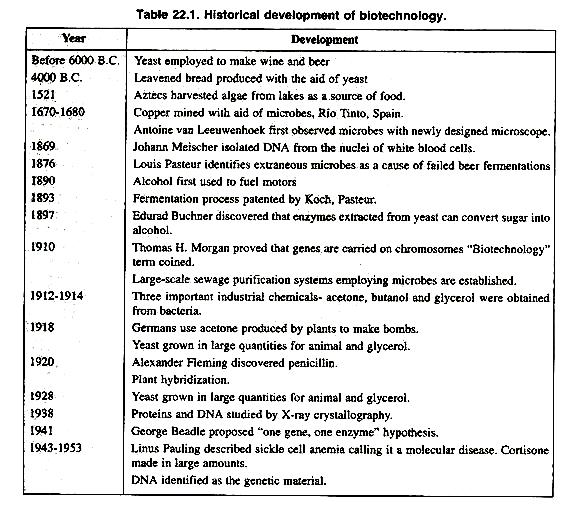
The document focuses on the development and application of modern biotechnology based on new enabling techniques of recombinant-DNA technology, often referred to as genetic engineering. The history of biotechnology begins with zymotechnology, which commenced with a focus on brewing techniques for beer. By World War I, however, zymotechnology would expand to tackle larger industrial issues, and the potential of industrial fermentation gave rise to biotechnology. The oldest biotechnological processes are found in microbial fermentations, as born out by a Babylonian tablet circa 6000 B.C. unearthed in 1881 and explaining the preparation of beer.
In about 4000 B.C. leavened bread was produced with the aid of yeast. The Sumerians were able to brew as many as twenty types of beer in the third millennium B.C. In the 14th century, first vinegar manufacturing industry was established in France near Orleans.
In 1680 Antony Van Leeuwenhoek first observed yeast cells with his newly designed microscope. In 1857, Louis Pasteur highlighted the lactic acid fermentation by microbe.
By the end of 19th century large number of industries and group of scientists were involved in the field of biotechnology and developed large scale sewage purification system employing microbes were established is Germany and France.
In 1914 to 1916, Delbruck, Heyduck and Hennerberg discovered the large-scale use of yeast in food industry. In the same period, acetone, butanol and glycerin were obtained from bacteria.
In 1920, Alexander Fleming discovered penicillin and large scale manufacturing of penicillin started in 1944. Table 22.1 presents chronological history of biotechnology.
Fermentation to Produce Foods:
Fermentation is perhaps the most ancient biotechnological discovery. Over 10,000 years ago mankind was producing wine, beer, vinegar and bread using microorganisms, primarily yeast. Yogurt was produced by lactic acid bacteria in milk and molds were used to produce cheese. These processes are still in use today for the production of modern foods. However, the cultures that are used have been purified and often genetically refined to maintain the most desirable traits and highest quality of products.
Industrial Fermentation:
In 1897 the discovery that enzymes from yeast can convert sugar to alcohol lead to industrial processes for chemicals such as butanol, acetone and glycerol. Fermentation processes are still in use today in many modern biotech organizations, often for the production of enzymes to be used in pharmaceutical processes, environmental remediation and other industrial processes.
Food Preservation:
Drying, salting and freezing foods to prevent spoilage by microorganisms were practiced long before anyone really understood why they worked or even fully knew what caused the food to spoil in the first place.
Quarantines:
The practice of quarantining to prevent the spread of disease was in place long before the origins of disease were known. However, it demonstrates early acceptance that illness could be passed from an infected individual to another healthy individual, who would then begin to have symptoms of the disease.
Selective Plant Breeding:
Crop improvement, by selecting seeds from the most successful or healthiest plants, to obtain a new crop having the most desirable traits, is a form of early crop technology. Farmers learned that using only the seeds from the best plants would eventually enhance and strengthen the desired traits in subsequent crops. In the mid-1860’s, Gregor Mendel’s studies on inheritable traits of peas improved our understanding of genetic inheritance and lead to practices of cross-breeding (now known as hybridization).
In last fifteen years progress have been made by microbiologists and genetic engineers, and we are hopeful to solve many fold problems of the present day, specially energy and food crisis to cater the need of growing population of the world. Mineral ore deposits are also becoming more scarce and expensive to recover from earth’s crust.
Microorganisms can be used to enhance to recovery of metals from low-grade ores and from effluents containing undesirable quantities of heavy metals or other toxins. When these technologies are applied at industrial level, they constitute bio-industry (Table 22.2).
2. Scope of Biotechnology:
Genetic engineering in biotechnology stimulated hopes for both therapeutic proteins, drugs and biological organisms themselves, such as seeds, pesticides, engineered yeasts, and modified human cells for treating genetic diseases. The field of genetic engineering remains a heated topic of discussion in today’s society with the advent of gene therapy, stem cell research, cloning, and genetically-modified food.
Biotechnology is the applied science and has made advances in two major areas, viz., molecular biology and production of industrially important bio-chemical. The scientists are now diverting themselves toward biotechnological companies; this has caused the development of many biotechnological industries.
In USA alone more than 225 companies have been established and successfully working, like Biogen, Cetus, Geneatech, Hybritech, etc. In world, USA, Japan, and many countries of Europe are leaders in biotechnological researchers encouraged by industrialists.
These companies are working for human welfare and opted following areas for research and development:
(a) Automated bio-screening for therapeutic agents.
(b) Bio-processing alkenes to valuable oxides and glycols.
(c) Developing immobilized cell and enzyme systems for chemical process industries.
(d) Engineering of a series of organisms for specific industrial use.
(e) Genetical improvement of microorganisms for production of pharmaceutical products.
(f) Human gene therapy.
(g) Improved production of Vitamin B12.
(h) Large-scale production of fructose from inexpensive forms of glucose.
(i) Manufacturing ethanol by continuous fermentation.
(j) Microbiological based production of human insulin and interferon’s.
(k) Microbiologically up-gradation of hydrocarbons.
(l) Production and development of vaccine to prevent calibacillosis.
(m) Production of bio-pesticide and bio-fertilizers.
(n) Production of diagnostic kits for toxoplasmosis identification.
(o) Production of monoclonal antibodies for organ transplant tissue typing.
(p) Production of photo-synthetically efficient plants.
(q) Production of transgenic plants and animals.
(r) Production of xanthan gum in oil fields for recovery of crude mineral oils.
The advances in recombinant DNA technology have occurred in parallel with the development of genetic processes and biological variations. The development of new technologies have resulted into production of large amount of biochemically-defined proteins of medical significance and created an enormous potential for pharmaceutical industries.
Biotechnology in itself is a vast subject and its scope is extended to various branches of biology. This includes plant tissue culture, production of transgenic in animal and plants, applications in medicine as tools and therapeutics, creation of new enzymes and their immobilization for industrial use, development of monoclonal antibodies and control of pollutions, etc.
3. Applications:
Industrial Applications of Biotechnology:
The industrial application of molecular biotechnology is often subdivided, so that we speak of red, green, gray or white biotechnology. This distinction relates to the use of the technology in the medical field (in human and animal medicine), agriculture, the environment and industry.
Some companies also apply knowledge deriving from molecular biotechnology in areas that cut across these distinctions (e.g., in red and green biotechnology, sequencing services). According to an investigation by Ernst and Young relating to the German biotech industry, 92% of companies are currently (2004) working in the field of red biotechnology, 13% in green, and 13% in gray or white biotechnology.
Biotechnology in Medicine:
Biotechnology products for therapeutic use include a very diverse range of products, as outlined in Tables 22.4, 22.5. Some products are intended to mimic the human counterpart, whereas others are intended to differ from the human counterpart and may be analogues, chemically modified (e.g., pegylated) or novel products (e.g., single chain or fragment antibody products, gene transfer vectors, tissue-engineered products).
Most of these products are regulated as medicinal products; however, the regulatory status of others such as some cell therapies and tissue: organ-based products differs globally and falls within the borderline between the practice of medicine, medical devices and medicinal products. Different areas of medicine in which biotechnology is used to develop diagnostic kits and cure are presented in the Figure 22.1.
Biotechnology-derived pharmaceuticals may be derived from a variety of expression systems such as Escherichia coli, yeast, mammalian, insect or plant cells, transgenic animals or other organisms. The expressed protein or gene may have the identical amino acid or nucleotide sequence as the human endogenous form, or may be intentionally different in sequence to confer some technical advantage such as an optimized pharmacokinetic or pharmacodynamics profile.
The glycosylation pattern of protein products is likely to differ from the endogenous human form due to the different glycosylation preferences of the expression system used. Furthermore, intentional post-translation modifications or alterations may be made such as pegylation. It is important for the toxicologist to be aware of the nature of the product to be tested in terms of primary, secondary and tertiary structure, and any post-translational modifications such as glycosylation status, particularly as these may be altered if the manufacturing system is modified.
Red Biotechnology:
Within the field of red biotechnology, which deals with applications in human and animal medicine, there are various further distinctions that can be made: biopharmaceutical drug development, drug delivery cell and gene therapies, tissue engineering/regenerative medicine, pharmacogenomics (personalized medicine), system biology, and diagnosis using molecular medicine.
Biopharmaceutical Drug Development:
In the field of biopharmaceutical drug development, it is the development of therapeutic human proteins by recombinant methods. (Table 22.5) for use as medicines that has the longest tradition. As mentioned above, recombinant human insulin was the first recombinant medicine in the world, produced by Genentech and brought to market in 1982. Today, recombinant human insulin has almost completely driven the other preparation of insulin (isolated from human or animal tissues) from the market.
The first therapeutic antibodies, especially monoclonal antibodies, have been on the market since the late 1990s. In 2002, antibodies were (along with vaccines) the most important therapeutic class of drugs under development and there are also more recent market studies more than 100 antibodies or antibody fragments were at the clinical development stage in 2002 and research and development is being carried out on around 470 more in about 200 companies around the world (Table 22.6,7).
Since the introduction of therapeutic antibodies onto the market, they have achieved significant turnovers, which are growing continually. The market for 2008 is estimated at a volume of US $16.7 billion (from Data-monitor, November 2003). Today, in addition to proteins, which currently play the most significant role in the biopharmaceutical field, new types of drugs based on RNA (antisense drugs, ribozymes, aptamers, Spiegelmers and RNA interference) are also being developed on the basis of advances in knowledge on molecular biotechnology.
Drug Delivery:
Closed linked to the development of therapeutic agents are the means of achieving their targeted delivery to their site of action. These drug delivery systems are mainly used for drugs whose physical and chemical characteristics make them insufficiently stable in reaching their site of action intact. They can also be used to transport drugs in a targeted way to particular sites of action (tissue specific targeting), or to overcome biological barriers such as the intestinal wall or the blood-brain barrier.
Green Biotechnology:
Green biotechnology is the application of biotechnology processes in agriculture and food production. The main dominant forces in green biotechnology today are agro giants with a worldwide area of operation such as BASF, Bayer Crop-Science, Monsanto and Syngenta. They are concentrating considerable attention on molecular plant biotechnology, which is seen as a future growth factor in agro-industry. The traditional pesticide market, on the other hand has been stagnating for years.
Transgenic Plants:
The main emphasis in modern plant biotechnology is the production of transgenic plants. The first use of gene technology to bring about changes in plants became possible at the beginning of the 1980s, around ten years after the first experiment with bacteria. The market value of transgenic plants is estimated to be in excess of 2 billion euros, according to the calculation of the German Federal Office for the Environment. These figures relate to transgenic crop plants, which were being grown on an area totaling about 40 million hectares worldwide in 1999 and 2000.
Novel and Functional food:
New types of foodstuffs with novel properties are often called functional food. Another category that is often mentioned in this context is nutraceuticals. These are foods that have a medicinal effect.
Livestock Breeding:
Modern biotechnology is being employed commercially to introduce novel performance features in productive livestock. The transgenic specimens then display for example different wool characteristics for sheep, or improved milk characteristics in cattle.
Grey/White Biotechnology:
The terms Grey and White Biotechnology have been coined for the application of biotechnological processes in environmental and industrial production contexts. The latter is primarily focused on the production of fine chemicals, in particular technical enzymes.
Technical Enzymes:
Modern biotechnology already dominates the technical enzymes market. They can be found as proteases, lipases, celluloses and amylases for example in modern detergents, where the serve, amongst other purposes as protein and fat solubilizes.
Safety Concerns:
There are a number of safety issues relating to biotechnology products that differ from those raised by low molecular weight products and need to be taken into account when designing the safety evaluation programme for a biotechnology derived pharmaceutical product.
The quality and consistency of the product requires careful control in terms of product identity, potency and purity because of concerns about microbiological safety, impurities arising from the manufacturing process (e.g., host-cell contaminants, endotoxin, residual DNA levels and process chemicals), and the fidelity of the protein sequence and post-translational modifications during process improvements and scale-up.
The immunogenic nature of heterologous proteins, vectors, cells, tissues and process contaminants must also be considered in the design of the safety evaluation programme and appropriate monitoring for anti-product antibodies, particularly neutralizing antibodies included in toxicity studies to aid interpretation of the findings. For gene transfer products, there are concerns about the distribution and persistence of vector sequences, the potential for expression of vector sequences in non-target cells: tissues and, in particular, the potential for inadvertent gonadal distribution and germ-line integration.
In 1997, the Food and Drug Administration (FDA) became aware that preclinical studies from multiple clinical trial applications indicated evidence of vector DNA in animal gonadal tissues following extra gonadal administration. These positive polymerase chain reaction (PCR) signals were for DNA extracts from whole gonads subsequent to vector administration. The observations involved multiple classes of vectors, formulations and routes of administration.