The below mentioned article provides notes on the Krebs’ Cycle.
The TCA cycle or Krebs’ cycle (after H. A. Krebs) is a cyclic sequence of reactions through which pyruvic acid produced in the EMP and EDP is oxidized. The cycle operates in aerobic organisms including animals, plants and microorganisms. The main function of the cycle is to generate energy by oxidation of acetic acid which is produced by decarboxylation of pyruvic acid and fed into the TCA cycle as acetyl-CoA.
In that sense, the cycle is a sequence of catabolic reactions. But, the TCA cycle also produces some intermediates which are utilized for biosynthesis of protein, nucleic acids etc. Thus the cycle serves a dual purpose, on one hand, a catabolic role and, on the other, an anabolic one. Such metabolic pathways serving dual purposes are known as amphibolic.
Pyruvic acid produced by breakdown of glucose is not fed into the TCA cycle as such but is converted to acetic acid by removal of one molecule of CO2. The reaction is catalysed by pyruvic acid decarboxylase and is a complicated one requiring thiamine pyrophosphate (TPP), lipoic acid and coenzyme A. Pyruvic decarboxylase is a multi-enzyme complex which in E. coli consists of three different enzymes — pyruvic decarboxylase (Enzyme 1), lipoic acid reductase- transacetylase (Enzyme 2) and di-hydro-lipoic acid dehydrogenase (Enzyme 3).
The sequential reactions catalysed by this multi-enzyme complex starts with binding of the substrate (pyruvic acid) to the TPP-containing Enzyme 1 (El) resulting in decarboxylation of pyruvic acid to hydroxyethyl-TPP. This product then reacts with the disulfide form of lipoic acid attached to Enzyme 2 (E2) to produce acetyl lipoic acid.
The acetyl group of this compound is transferred to free coenzyme A (HS-CoA) to produce acetyl CoA. Lipoic acid, released in reduced form from the last transfer reaction is oxidized by Enzyme 3 (E3) in which NAD acts as H-acceptor.
The pyruvic acid decarboxylation reaction is shown in Fig. 8.46.
So only the relevant portions of these compounds are shown in the figure:
Step 1:
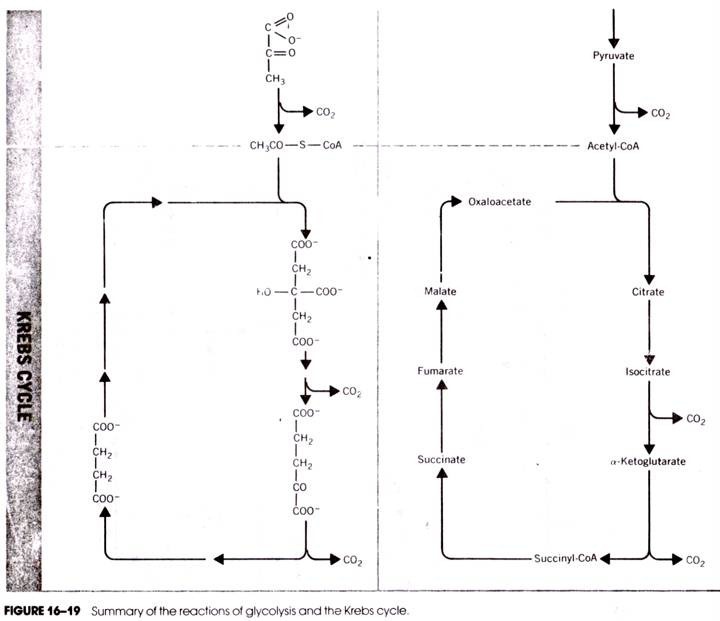
The reactions of TCA cycle starts by condensation of acetyl-CoA with oxalacetic acid (OAA) catalysed by the citrate synthetase producing citric acid. In this reaction, the keto group of OAA condenses with the acetyl-group of acetyl-CoA resulting in the hydrolysis of the thioester bond and liberation of free coenzyme A in SH-form.
Steps 2 and 3:
The next two steps are catalysed by the enzyme aconitase which reversibly adds water to form cis-aconitic acid and isocitric acid. Citric acid, cis-aconitic acid and isocitric acid exist in an equilibrium in which the major portion (-91%) remains as citric acid, 3% as cis-aconitic acid and 6% as isocitric acid.
Steps 4 and 5:
These two steps involve dehydrogenation of isocitric acid to oxalsuccinic acid which is then decarboxylated to yield α-keto glutaric acid. Both the steps are catalysed by the same enzyme, isocitric acid dehydrogenase which can use either NAD or NADP as coenzyme.
Steps 6 and 7:
In these two steps, α-ketoglutaric acid is decarboxylated and simultaneously dehydrogenated first to succinyl CoA and then to succinic acid. Thus, these two steps constitute oxidative decarboxylation which is similar to that of decarboxylation of pyruvic acid to acetyl-CoA. The enzyme concerned is α-keto glutarate dehydrogenase.
It is a multi-enzyme complex requiring the same coenzymes as pyruvic decarboxylase, viz. TPP, lipoic acid, NAD and coenzyme A. Oxidative decarboxylation of α-ketoglutaric acid produces one molecule of energy-rich compound in the form of GTP (guanosine triphosphate) through the action of the enzyme, succinyl-thiokinase which catalyses the second step.
In the α-ketoglutarate dehydrogenase reaction, the enzyme-bound TPP combines with substrate and decarboxylates it. The enzyme-bound decarboxylated product is then transferred to lipoic acid and TPP is set free.
The succinyl rest is then taken up by HS-CoA, setting lipoic acid free and producing succinyl-CoA. The high energy thioester bond of succinyl-CoA is utilized for phosphorylation of GDP to GTP by the action of the enzyme succinyl thiokinase. GTP can form ATP by reacting with ADP by a nucleoside di-phosphokinase reaction.
Step 8:
This step consists of dehydrogenation of succinic acid to fumaric acid catalysed by succinate dehydrogenase, FAD acting as a coenzyme. Unlike NAD, FAD is covalently bound to the apoenzyme forming a flavoprotein.
Step 9:
In this step, fumaric acid is hydrated through the action of fumarase producing malic acid.
Step 10:
In this final step of TCA cycle, malic acid is oxidized to oxalacetic acid by the NAD-linked enzyme malate dehydrogenase. Oxalacetic acid so formed then accepts another molecule of acetyl- CoA to initiate another turn of the TCA cycle.
The TCA is shown in Fig 8.47:
Summing up the TCA cycle, it may be observed that through one complete turn of the cycle, the two C-atoms that enter the TCA cycle from acetyl-CoA are oxidized to CO2 and the H-atoms of acetyl- group are transferred to coenzymes, NAD and FAD.
The decarboxylation reactions are in step 5 and step 6 in which the 6-carbon compound, oxalsuccinic acid is converted to the 5-carbon compound, α-ketoglutaric acid, and the latter to the 4-carbon compound, succinic acid.
The dehydrogenation reactions are in Steps 4, 6, 8 and 10 through which four pairs of H-atoms are transferred — three to NAD and one pair to FAD. Also, through one turn of the cycle, one molecule of an energy-rich compound, viz. GTP, is formed which is equivalent to one ATP.
The overall reaction of TCA cycle can, therefore, be written as:
Acetyl-CoA + 3H2O + 3NAD + 1FAD + GDP + Pi -> 2CO2 + HS-CoA + 3NADH2 + 1FADH2 + 1GTP.
Although the TCA cycle is amphibolic in nature — which means it is both catabolic and anabolic — it is the main pathway for oxidizing substrates for energy generation in all aerobically respiring organisms by transfer of the H-atoms to the electron transport system (ETS). It is important to note that the TCA cycle enzymes and the ETS, both, are located in the mitochondria in case of eukaryotic organisms and in the cell membrane in case of prokaryotic cells.
The Glyoxylate Cycle:
The glyoxylate cycle is also known as glyoxylate shunt because it is a bypass of the TCA cycle. This cycle is absent in animals, but it occurs in microorganisms when they are grown on acetate or fatty acids as sole carbon source. The cycle also operates in oilseed plants during seed germination when the stored oil is utilized for energy production.
In addition to the enzymes of the TCA cycle, this pathway has two extra enzymes. One Of these two is isocitrate lyase (also called isocitratase) which cleaves isocitric acid into succinic acid and glyoxylic acid. The other enzyme is malate synthetase which catalyses the condensation of glyoxylic acid and acetyl-CoA to form malic acid with liberation of free coenzyme A.
Succinic acid produced by the lyase is converted to fumarate and malate by the usual TCA cycle enzymes. The glyoxylate pathway utilizes two acetyl-CoA molecules, one in formation citric acid from oxalacetic acid and the other in formation of malic acid from glyoxylic acid. Thus, the acetyl-CoA derived from fatty acid oxidation can be more efficiently used through this pathway in comparison to the TCA cycle.
An outline of the glyoxylate cycle is presented in Fig. 8.48:
The enzymes of the glyoxylate bypass, viz. isocitrate lyase and malate synthetase, are located not in the mitochondria but in a different type of membrane-bound organelles in plant cells, called the glyoxysomes. They also differ from mitochondria by not having cytochromes.
Ancillary or Anaplerotic Reactions:
The TCA cycle also provides intermediates for biosynthesis of several metabolites. This means that such intermediates are continuously withdrawn from the cycle, causing a shortfall in the regeneration of the acceptor of acetyl-CoA i.e. oxalacetic acid.
Some special enzymatic reactions exist to replenish the shortfall of intermediates of the TCA cycle. These reactions are called anaplerotic or ancillary. The most important of these reactions are those which replenish oxalacetic acid by adding CO2 to a three-carbon compound, like pyruvic acid or phosphoenol pyruvic acid. A number of such reactions catalysed by different enzymes have been reported. One of such carboxylation reactions is catalysed by pyruvic acid carboxylase which converts pyruvic acid to oxalacetic acid.
These are described below:
The enzyme was discovered by Wood and Werkman and the reaction is named after them as Wood and Werkman Reaction. They were the first to demonstrate that CO2-fixation is not restricted exclusively to autotrophic organisms, but the phenomenon also occurs in heterotrophs. This mode of CO2-fixation in heterotrophic organisms is known as dark-fixation in contrast to the mode of CO2-fixation in phototrophic organism.
The enzyme pyruvic acid carboxylase requires biotin as a cofactor. In fact, biotin combines with CO2 in presence of ATP to form carboxy-biotin or “active CO2” which transfers CO2 to the acceptor which is, in this case, pyruvic acid to form oxalacetic acid.
The enzyme is activated in presence of acetyl-CoA which acts as a positive modulator. When excess of acetyl-CoA accumulates in the cell, it accelerates the enzyme, so that more oxalacetic acid may be formed to accept more of acetyl-CoA, thereby reducing the concentration of accumulated acetyl CoA.
Another enzyme serving the formation of oxalacetic acid is phosphoenol pyruvic acid carboxylase which catalyses conversion of phosphoenol pyruvic acid to oxalacetic acid. No ATP is required, because PEP itself is an energy-rich compound. The enzyme occurs in plants and bacteria.
A third enzyme, called phosphoenol pyruvic carboxykinase, also catalyses carboxylation of PEP to oxalacetic acid. The enzyme occurs in animal systems. This enzyme can transfer the energy-rich phosphate group of PEP to GDP or IDP (inosine diphosphate) producing GTP or ITP.