In this article we will discuss about the molecular structure of lipids.
Lipids are another group of important biological molecules present in all cellular organisms and some viruses. Unlike the other groups of biological molecules, lipids are highly heterogenous in chemical structure. But all of them possess a common physical property which is that they are all non-polar compounds, insoluble in water and soluble in organic solvents like ether, chloroform etc.
Lipids form a major component of all types of biological membranes. The cytoplasmic membrane which is the limiting layer separating the biological cell from the outer environment is present universally in all organisms. It consists mainly of a lipoprotein bilayer. In eukaryotic cells various compartments or cell organelles are also separated by membrane system constituting the cytoskeleton.
On the basis of chemical structure and constitution, lipids are broadly classified into two categories:
i. The simple lipids and
ii. The complex lipids.
(i) Simple Lipids:
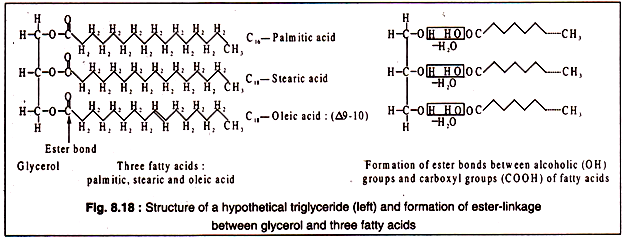
Simple lipids contain a trihydric alcohol, glycerol and long chain fatty acids. The carboxyl groups of the fatty acids are ester-linked to the hydroxyl groups of glycerol. The fatty acids present in simple lipids have generally 16 or 18 carbon atoms and they may be saturated or unsaturated. The unsaturated fatty acids, usually have one or two double bonds. Such lipids having three molecules of fatty acids esterified to glycerol are called triglycerides.
The structure of a triglyceride and the formation of ester- bond are shown in Fig. 8.18:
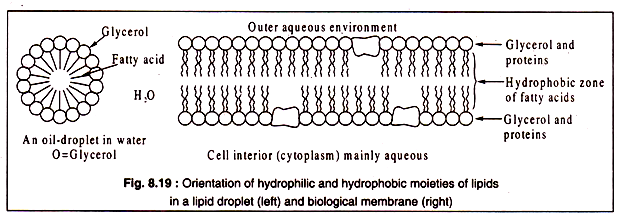
It is important to note that the hydrophobic nature of fats and oils is due to the long-chain fatty- acids which are highly insoluble in water and are strongly hydrophobic, though glycerol itself is a hydrophilic compound. When oil or fat is dispersed in water, they form an unstable emulsion consisting of small droplets of oil or fat.
In these droplets, the lipid molecules are oriented in such a manner that the hydrophilic glycerol moieties remain in contact with water, while the fatty acid tails project inward to build a compact hydrophobic central zone.
The same principle applies also in the formation of biological membranes in which the hydrophilic glycerol and protein molecules form a layer towards the outside and the lipid-tails remain inside. Thus, in a bilayered membrane, lipids form the middle hydrophobic layer and glycerol and protein remain on two sides facing the outer and inner aqueous environment.
This is diagrammatically represented in Fig. 8.19:
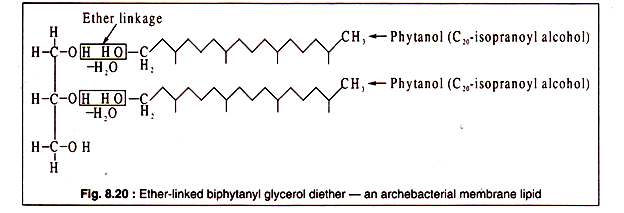
Although ester-linked lipids are present in most organisms, an exceptional type of lipids occurs in the membranes of archaebacteria and in some primitive eubacteria. The lipids in these organisms are ether-linked. An ether-bond is formed when two alcoholic (OH) groups react with elimination of a molecule of water.
In arch bacterial lipids, the hydroxyl groups of glycerol and long-chain alcohols like phytanol produce ether bonds as shown in Fig. 8.20:
(ii) Complex Lipids:
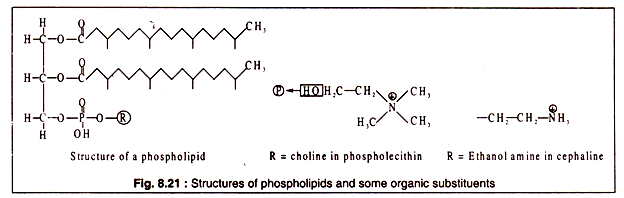
In contrast to simple lipids, the complex lipids contain elements like phosphorus, sulfur, nitrogen etc., besides carbon, hydrogen and oxygen which are present in all lipids. Among the complex lipids, phospholipids resemble the simple lipids most closely in their structure.
Phospholipids are a major constituent of the cell membranes of most of the organisms. In a phospholipid molecule, two hydroxyl groups of glycerol are esterified with carboxyl groups of long chain fatty acids as in case simple lipids, while the third hydroxyl group of glycerol is esterified with phosphoric acid. Such a lipid is called a phosphatide. In most of the phospholipids, phosphoric acid is further linked to an organic group (R).
The structures of some phospholipid are shown in Fig. 8.21:
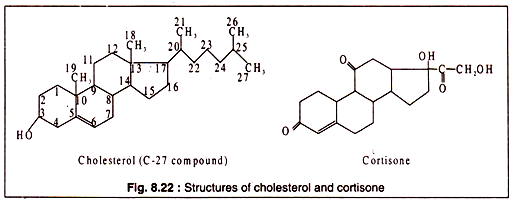
Another group of complex lipids are steroids which are quite different in chemical structure from simple lipids. The steroid nucleus contains three 6-membered and one 5-membered carbon rings. Steroids having an alcoholic (OH) group attached to one of the rings are known as sterols, e.g. cholesterol. Sterols are widely distributed in the plasma membranes of animals, plants and fungi, but not in bacteria (except in one group lacking a cell wall — the mycoplasmas).
In the membranes, sterols are disposed in between the fatty acid chains of lipid bilayer and they seem to prevent the compactness of the membrane under low temperature conditions. The structure of cholesterol is shown in Fig. 8.22. Many animal hormones, like oestrogen, androsterone, progesterone etc. are steroids. Cortisone is an important steroid drug (Fig. 8.22).
A group of complex lipids, called mycotic acids, are present in the cell walls of some Gram-positive bacteria, like mycobacteria, corynebacteria and some nocardia forms. Mycolic acids are high molecular lipids, called waxes, consisting of very long fatty acids with 60 to 90 carbon atoms. The presence of mycolic acids in these bacteria makes them acid-fast.
Many other complex lipids of diverse chemical compositions are present in living organisms, particularly animals. Some of these can be briefly mentioned as – Sphingolipids consisting of an 18-C amino alcohol, sphingosine as the basic unit and choline are richly represented in the nerve and brain tissues. Cerebrosides are more or less similar lipids with galactose instead of choline. Gangliosides are also glycolipids found in nerve ganglia. They contain a sphingosine joined to fatty acids and carbohydrates.
The structure of sphingosine is shown in Fig. 8.23: