In this article we will discuss about the biosynthesis of murein.
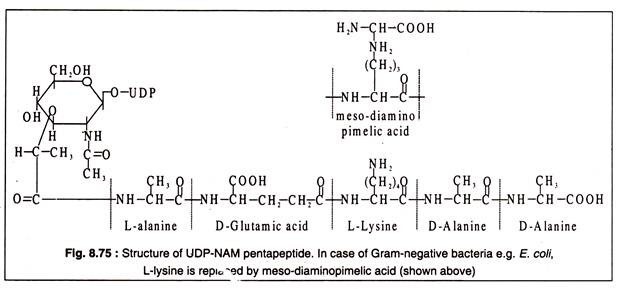
A characteristic polymer that forms the backbone of all eubacterial cell wall is peptidoglycan or murein. Murein essentially consists of parallelly running polysaccharide chains, the repeating unit of which is a disaccharide of N-acetyl-glucosamine (NAG) and N-acetyl muramic acid (NAM) having a tetra-peptide bonded to its lactyl group. The tetra-peptide side chain contains L-alanine, D-alanine, D-glutamic acid and either meso-diaminopimelic acid or its decarboxylated product, L-lysine.
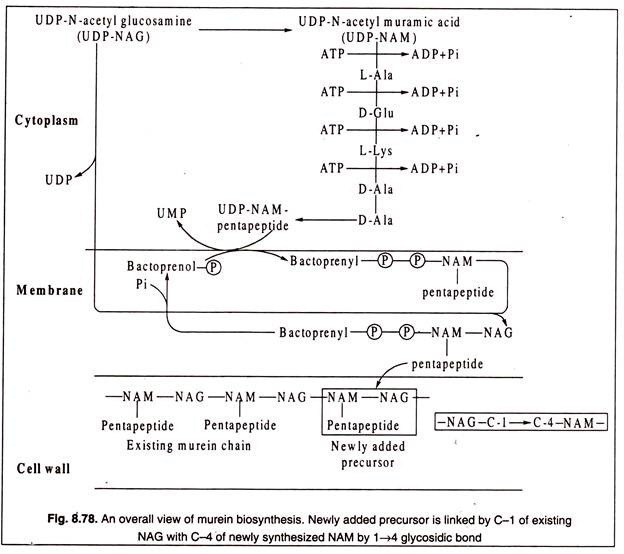
Biosynthesis of murein starts in the cytoplasm and then the precursors are transported to the cell membrane where further synthesis continues. Finally, the precursors are transported across the cell membrane into the periplasmic space in case of Gram-negative bacteria, or outside the membrane in case of Gram-positive bacteria and incorporated into the growing murein chain.
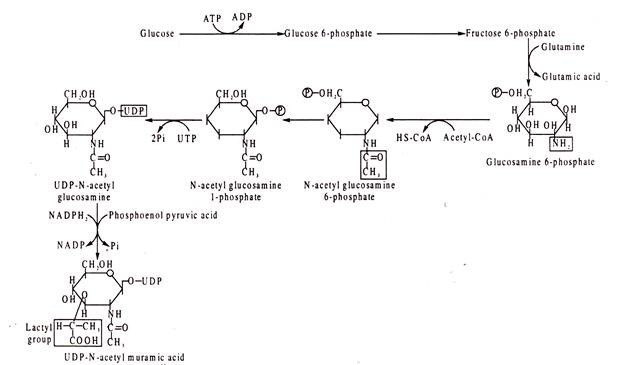
The biosynthesis of murein begins with addition of a lactyl-group donated by phosphoenol pyruvic acid to N-acetyl glucosamine resulting in formation of N-acetyl muramic acid. Prior to this step, N-acetyl glucosamine is produced from glucose in several steps. It is to be noted that in these biosynthetic processes UDP (uridine diphosphate) acts as a carrier.
NAG in its phosphorylated form is transferred to UTP to form UDP-NAG which is next converted to NAM:
To the UDP-N-acetyl muramic acid (UDP-NAM) so formed are added the amino acids, one by one, to build first a tripeptide side chain and then a pentapeptide side chain. The peptide chain is linked to the lactyl group of UDP-NAM, so that the α-amino group of the first amino acid, L-alanine, is linked with the carboxyl group of lactyl moiety by a peptide bond. The successive amino acids are then linked by peptide bonds with the preceding amino acid. To the tripeptide chain so formed is added a dipeptide, D-alanyl-D-alanine to complete the pentapeptide side chain of UDP-NAM (Fig. 8.75).
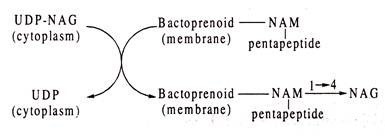
Up to the stage of synthesis of UDP-NAM-pentapeptide, the reactions occur in the cytoplasm of the bacterial cells. For the following reactions of the next stage, the UDP-NAM-pentapeptide is transferred to a membrane-bound phospholipid with liberation of UDP. This phospholipid, known as bactoprenol, is undecaprenyl phosphate, a terpenoid having 55 C-atoms and a phosphate group.
Its structure is shown below (Fig. 8.76):
Next, a disaccharide is formed by joining UDP-NAG with the bactoprenoid NAM-pentapeptide by 1——–> 4 glycosidic linkage and setting in this reaction UDP free. The formation of disaccharide takes place in the membrane.
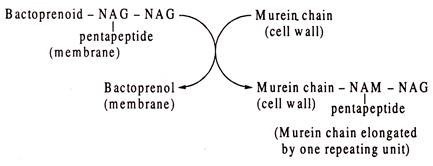
At the next step, the disaccharide with attached pentapeptide is transported outside the membrane leaving the bactoprenoid carrier in the membrane. The disaccharide unit acts as a precursor and is added to the murein chain in the cell wall. Thus, accretion of new cell wall material occurs by transfer of newly synthesized disaccharide-pentapeptide units from bactoprenoid carriers across the membrane to the existing peptidoglycan (murein) chain in the cell wall. By each transfer, the chain is extended by a disaccharide unit (NAG-NAM-pentapeptide) at its reducing end (i.e. C-l of NAG or NAM).
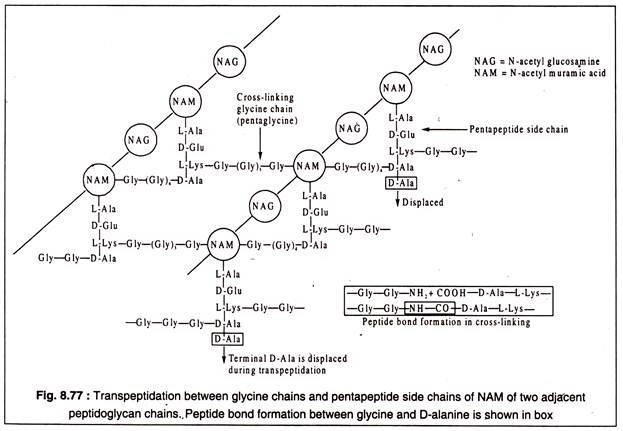
The next step of murein biosynthesis consists of cross-linking of the pentapeptide side chains by addition of short chains of amino acids which connect L-lysine of one pentapeptide chain with the penultimate D-alanine of another pentapeptide, thereby displacing the terminal D-alanine which is set free. In case of Micrococcus lysodeikticus the cross-linking peptide consists of 5 glycine molecules.
The cross-linking of pentapeptide side chains of NAM is called transpeptidation. Several antibiotics, like penicillins, vancomycin, novobiocin, bacitracin etc. are known to inhibit transpeptidation. Another antibiotic, D-cycloserine, inhibits the formation D-alanyl-D-alanine and, hence, the dipeptide cannot be incorporated into pentapeptide. Transpeptidation between parallelly running adjacent peptidoglycan chains is diagrammatically represented in Fig. 8.77.
In presence of low concentration of penicillin, the bacteria excrete uridine nucleotides, known as Park nucleotides (after the discoverer), consisting of UDP-N-acetyl muramic acid and varying lengths of amino acid chain. This indicates that polymerization of murein in the cell wall occurs through transpeptidation of newly synthesized precursors. Because penicillin inhibits transpeptidation, the newly synthesized precursors cannot be incorporated into the peptidoglycan net.
An overall picture of biosynthesis of murein is presented in Fig. 8.78: