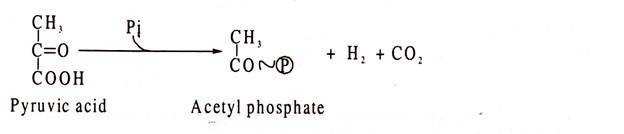The following points highlight the five main types of fermentation. The types are: 1. Alcoholic Fermentation 2. Lactic Acid Fermentation 3. Propionic Acid Fermentation 4. Butyric Acid — Butanol Fermentation 5. Mixed Acid Fermentation.
Type # 1. Alcoholic Fermentation:
Alcoholic fermentation generally means production of ethanol (CH3CH2OH). Commonly yeasts, particularly Saccharomyces cerevisiae, are used for production of various alcoholic beverages, as well as industrial alcohol. Yeasts are essentially aerobic organisms, but they can also grow as facultative anaerobes.
The energy-yield under anaerobic conditions is much lower and hence the growth is slower with much lower cell-yield. When grown with aeration, the cell-yield increases dramatically, but alcohol production falls. Thus, oxygen inhibits fermentation. This is known as Pasteur-effect.
Conversion of pyruvic acid to ethanol proceeds in two steps: pyruvic acid to acetaldehyde and acetaldehyde to ethanol. The first step is catalysed by pyruvic acid decarboxylase which requires TPP as coenzyme, and the second step by alcohol dehydrogenase which requires NADH2 as coenzyme.
NADH2 is thereby oxidized to NAD which can be reused for reduction of GAP to DPGA in the EMP:
Various strains of yeasts, mostly belonging to Saccharomyces cerevisiae, have been developed and carefully selected for large-scale manufacture of alcohol for different purposes. Also, various materials and conditions are used depending on the nature of the product desired.
For example, for production of baker’s yeast used in bread industry, strongly aerated cultures favour large cell-yield with little or no alcohol. Extract of malted (partly germinated) barley serves as substrate for beer production.
The starting material contains large amount of maltose (a dissacharide of two glucose units) produced by hydrolysis of starch present in barley seeds. Maltose is split into glucose and serves as substrate for alcohol fermentation under anaerobic conditions.
Similarly, for production of wine, grape juice is the substrate of choice. Specific selected strains are employed to impart characteristic flavour and taste of different alcoholic beverages. For manufacture of industrial alcohol, generally molasses is used as the starting material. Also sulfite liquor, which is a waste product of paper industry, is used as a cheap substrate for industrial alcohol production.
Besides yeasts, some bacteria can also carry out alcoholic fermentation. A well-known example is Zymomonas mobilis. This organism dissimilates glucose by EDP producing pyruvic acid which is converted to ethanol by decarboxylation and dehydrogenation as in yeast. Pseudomonas saccharophila is another bacterium which is used in alcoholic fermentation.
Type # 2. Lactic Acid Fermentation:
Lactic acid fermentations are of two types:
Homo-fermentative and
Heterofermentative.
In the first type, lactic acid is produced as the sole product by reduction of pyruvic acid with the help of the enzyme lactic acid dehydrogenase. The reaction regenerates NAD from NADH2 which is reused for oxidation of GAP to DPGA in the glycolytic pathway.
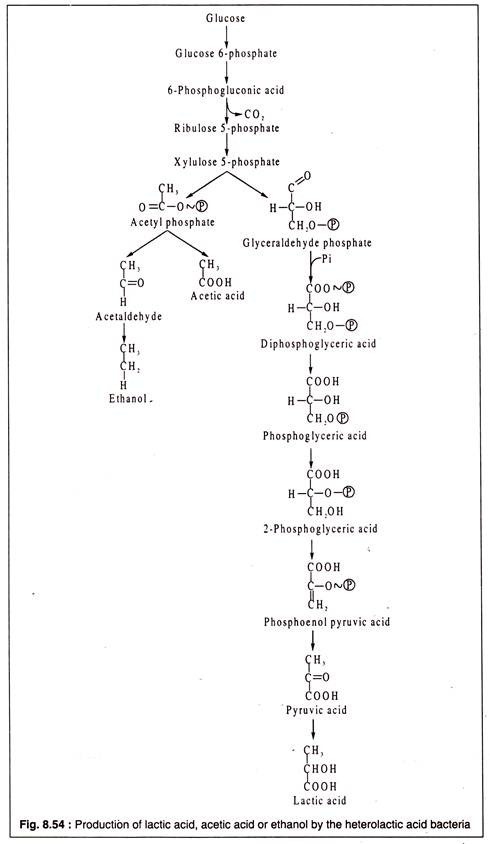
As one molecule of lactic acid is formed from one molecule of pyruvic acid, two molecules of lactic acid are produced from each molecule of glucose, when it is dissimilated through EMP. In heterofermentative type, the products are lactic acid and ethanol or acetic acid and CO2. The heterofermentative lactic acid bacteria dissimilate glucose via PPC. They produce lactic acid from one-half of the glucose molecule, and ethanol or acetic acid and CO2 from the other half.
Lactic acid bacteria are both morphologically and physiologically diverse. The lactic cocci, previously included in the genus Streptococcus, have been transferred to the genus Lactococcus. The rod-shaped lactic acid bacteria are distributed in several genera, though majority are placed in the genus Lactobacillus. Some representative species of homo-fermentative lactic acid bacteria are Lactococcus lactis, L. cremoris, L. diacetilactis, L. thermophilus, Lactobacillus lactis, L. bulgaricus, L. acidophilus etc. Representatives of heterofermentative type include Lenconostoc mesenteroides, Lactobacillus brevis, Bifidobacterium bifidum etc. There is also a spore-forming lactic bacterium, Sporolactobacillus. The lactic acid bacteria prefer anaerobic conditions for optimal growth as they do not have cytochromes or catalase, though they can also grow in microaerophilic environment.
Homolactic fermentation is the simplest of all fermentations, involving only a single step in which pyruvic acid is reduced to lactic acid. Lactic acid is formed also in muscles by a similar reaction.
The heterofermentative lactic acid bacteria lack two vital enzymes of the glycolytic pathway — aldolase and triose phosphate isomerase. Hence, they are unable to use EMP. As an alternative, they employ the pentose phosphate pathway. An intermediate of this pathway is xylulose 5-phosphate.
The heterofermentative bacteria cleave xylulose 5-phosphate by a TPP-linked pentose phosphate ketolase into glycerin aldehyde phosphate (GAP) and acetyl phosphate. GAP is then converted to pyruvic acid by the usual EMP enzymes, while acetyl phosphate is reduced either to acetic acid or to ethanol. From pyruvic acid, lactic acid is formed by the lactate dehydrogenase activity.
Leuconostoc mesenteroides produces from one molecule of glucose, one molecule of lactic acid, one molecule of ethanol and one molecule of CO2. On the other hand, Lactobacillus brevis produces acetic acid in place of ethanol.
The heterofermentative pathway is shown in Fig. 8.54:
Lactic acid bacteria are widely used for production of various fermented food throughout the world. The bacteria ferment the milk sugar (lactose) to produce lactic acid which curdles milk protein. Various species are used to yield products of variable consistency, taste and aroma. In different countries the products are variously known as yogurt in Europe and America, dadhi or dahi in India, Kefir in Russia, Kumiss, butter milk, acidophilus milk etc.
Lactic acid bacteria are also employed in producing fermented vegetable products, like sauerkraut (fermented cabbage), cucumber pickles and fermented olive. These bacteria are also used for production of sausages from beef and pork.
Both heterofermentative and homo-fermentative lactic acid bacteria are used as ‘starter’ for production of fermented food. Some of these bacteria are Lactococcus cremoris, L. lactis, L. thermophilus, Lactobacillus bulgaricus, L. plantarum, L. brevis, Leuconostoc mesenteroides, Pediococcus cerevisiae etc.
Type # 3. Propionic Acid Fermentation:
Propionic acid (CH3-CH2-COOH) is produced by several anaerobic bacteria among which are the coryneform Propionibacterium, and Veillonella, Clostridium, Selenomonas etc. Propionibacterium acidipropionici and P. freudenreichii are the main propionic acid fermenters. Propionibacteria possess cytochromes and catalase and can tolerate some amount of oxygen. They are natural inhabitants of rumen of herbivorous cattle.
The propionic acid bacteria dissimilate glucose via EMP and produce pyruvic acid. By a biotin- linked carboxylation reaction pyruvic acid is converted to oxalacetic acid which is then reduced in two steps to succinic acid through reversal of TCA cycle reactions.
Succinic acid is then converted to succinyl-CoA, also by a reverse step of the TCA cycle. Next, succinyl-CoA produces methyl malonyl- CoA by the action of a vitamin B12-linked enzyme methyl malonyl mutase which catalyses an intra-molecular rearrangement. Methyl malonyl-CoA is then decarboxylated to propionyl-CoA.
In the final step, propionyl-CoA yields propionic acid, and CoA is transferred to succinic acid by an enzyme, CoA-transferase. The pathway of propionic acid is shown in Fig. 8.55. Together with lactic acid bacteria, the propionic acid bacteria are used for commercial production of Swiss cheese. Propionic acid contributes to the special flavour of this cheese.
Type # 4. Butyric Acid — Butanol Fermentation:
The bacteria carrying out butyric acid-butanol fermentation are all obligately anaerobic spore- forming bacteria belonging to the genus Clostridium. Besides butyric acid and n-butanol, several other products of this fermentation are acetic acid, ethanol, isopropanol and acetone depending on species.
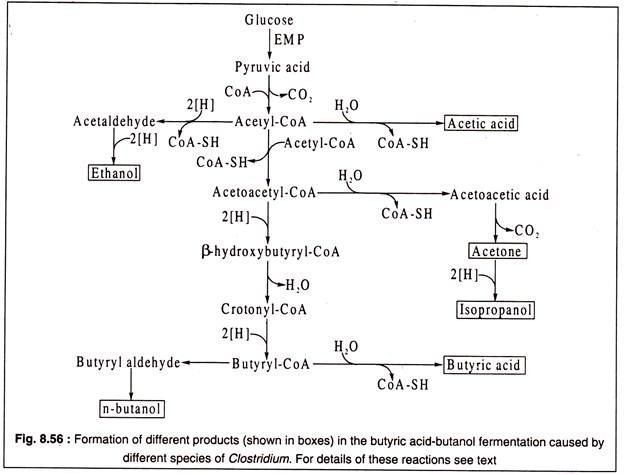
For example, C. butyricum, C. lactoacetophilum, C. pasteurianum etc. produce butyric acid together with acetic acid, while C. butylicum and C. acetobutylicum produce butyric acid, acetic acid and isopropanol or acetone. Also, as a fermentation product, CO2 is always present.
Clostridia dissimilate glucose by the EMP to form pyruvic acid which by decarboxylation produces acetyl-CoA.
The latter acts as the key intermediate in the butyric-butylic fermentation and gives rise to all the products by different pathways as shown in Fig. 8.56:
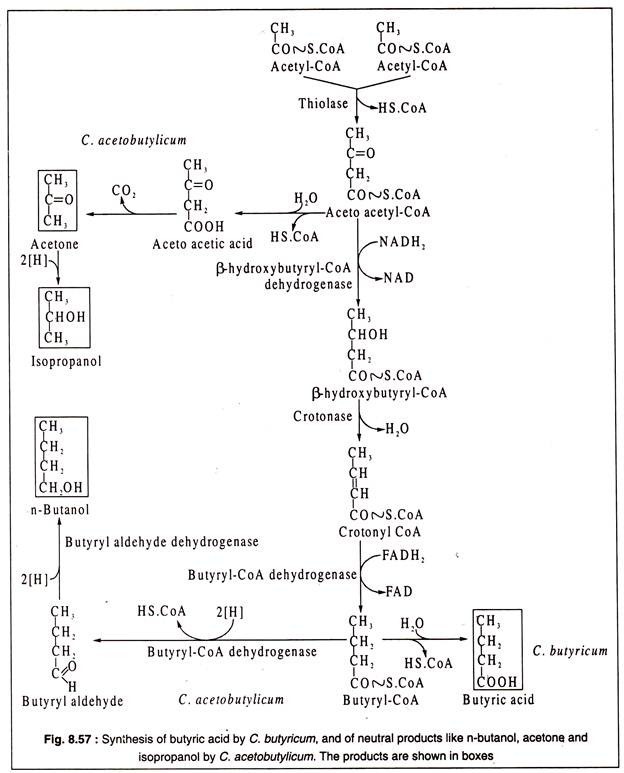
In the pathway leading to butyric acid in C. butyricum, two molecules of acetyl-CoA are condensed by the action of the enzyme thiolase to produce acetoacetyl CoA with liberation of one CoA. Acetoacetyl CoA is then dehydrogenated by β-hydroxybutyryl-CoA dehydrogenase to form P-hydroxbutyryl CoA with NADH2 acting as H-donor.
The dehydrogenated product next gives rise to crotonyl-CoA through the action of the enzyme crotonase. Crotonyl-CoA undergoes another step of reduction catalysed by butyryl-CoA dehydrogenase which is FADH2-linked producing butyryl-CoA. The latter is finally converted to butyric acid by removal of CoA and addition of water (Fig. 8.57).
Under alkaline conditions, butyryl CoA is converted by C. acetobutylicum to n-butanol through two steps catalysed by butyryl-CoA dehydrogenase and butyryl aldehyde dehydrogenase as shown Fig. 8.57.
C. acetobutylicum also produces isopropanol by reduction of acetone under alkaline conditions. Acetone is produced by decarboxylation of aceto acetic acid as shown in the figure 8.57.
Clostridia always produce molecular hydrogen as one of the fermentation products. Hydrogen originates from phosphoroclastic cleavage of pyruvate.
This cleavage produces acetyl phosphate, molecular hydrogen and CO2 as shown:
During such cleavage, hydrogen is at first transferred to an iron-containing protein called ferredoxine which is thereby reduced. Molecular hydrogen is liberated from the reduced compound through the action of hydrogenase, and ferredoxine is oxidized.
Type # 5. Mixed Acid Fermentation:
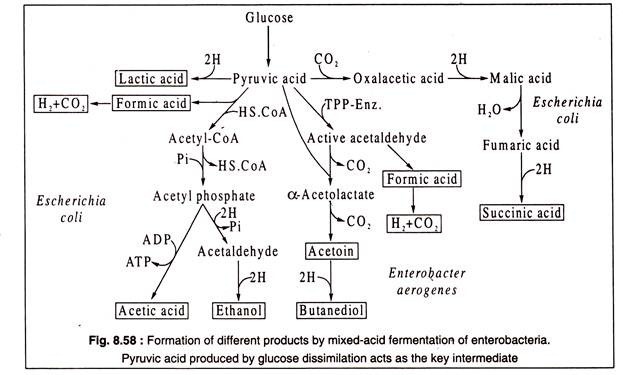
This type of fermentation occurs characteristically in bacteria belonging to the family Enterobacteriaceae. These bacteria can grow both aerobically carrying out oxygen respiration or anaerobically carrying out fermentation. The type of fermentation is called mixed-acid, because, as products, several different organic acids and neutral compounds are produced. A characteristic acid of mixed fermentation is formic acid, though it is by no means the major product.
Depending on species, a number of-different substances are formed, like acetic acid, succinic acid, lactic acid, ethanol, acetoin, butanediol, CO2 and molecular hydrogen. On the basis of fermentation products, the enterobacteria can be divided into two groups: one group having an Escherichia coli-type fermentation, and the other having an Enterobacter aerogenes type. One very significant difference in these two types is the formation of acetoin and butanediol (2, 3-butylene glycol) from pyruvic acid by Enterobacter aerogenes. In E. coli type of fermentation these are absent. Both types dissimilate glucose to pyruvic acid.
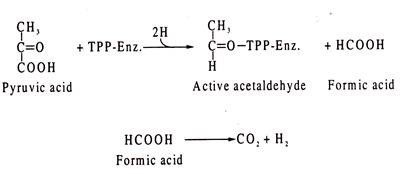
Mixed acid fermentation is sometimes called formic acid fermentation. Under anaerobic condition, E. coli cleaves pyruvic acid to acetyl-CoA and formic acid.
The reaction is catalysed by the enzyme, pyruvate-formic acid lyase as shown:
Formic acid so formed is then cleaved by another lyase, formic acid-hydrogen lyase to molecular hydrogen and CO2 which are liberated in gaseous form.
Formic acid is also produced in Enterobacter-type of fermentation, but in a different way. The reaction is catalyzed by a TPP-linked enzyme. In this type, pyruvic acid is cleaved into TPP-linked “active” acetaldehyde (hydroxyethyI-Tpp.Enz.) and formic acid.
Formic acid then breaks into CO2 and H2:
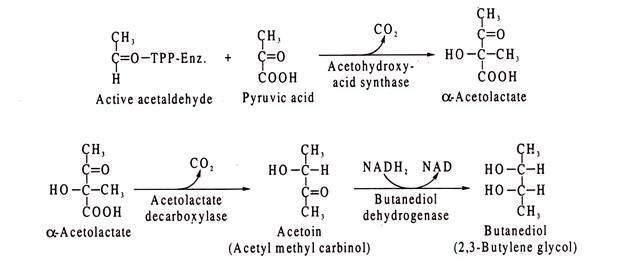
Enterobacter-type of fermentation produces acetoin (acetylmethyl carbinol) and butanediol which are not formed by E. coli-type of fermentation. The detection of acetoin and butanediol forms the basis of Voges-Proskauer reaction. Hence, E. coli is Voges-Proskauer negative.
Formation of acetoin and butanediol in Enterobacter proceeds via acetolactate pathway. The TPP- linked active acetaldehyde produced from pyruvic acid, described above, reacts with another molecule of pyruvic acid to form acetolactate.
The reaction is catalysed by acetohydroxyl acid synthase. Acetolactate so formed, is then decarboxylated by the enzyme acetolactate decarboxylase to produce acetoin. The latter is reduced by butanediol dehydrogenase to 2,3-butylene glycol (butanediol), NADH2 acting as H-donor.
The reactions are:
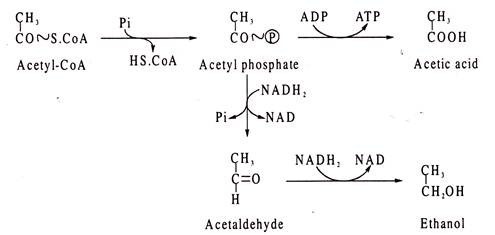
Other products of enteric bacteria fermentations include acetic acid, ethanol, lactic acid and succinic acid. AcetyI-CoA produced in pyruvic acid-formic acid lyase reaction in E.coli can be used in several ways.
It can be converted acetyI phosphate and from it either ethanol may be produced via acetaldehyde or it may form acetic acid as shown:
Lactic acid is formed directly from pyruvic acid through the action of lactate dehydrogenase. Succinic acid is produced also from pyruvic acid by carboxylation with the help of a biotin enzyme to oxalacetic acid. The latter leads to formation of succinic acid by reversal of steps of the TCA cycle.
Formation of different products of mixed-acid fermentations is summarized in Fig 8.58: