In this article we will discuss about competitive and non-competitive reversible enzyme inhibition.
Competitive Inhibition:
The main feature of competitive inhibition is that it can be reversed by increasing the substrate concentration in a reaction mixture which contains both the substrate and the inhibitor. The degree of inhibition depends on the relative concentrations of the substrate and the inhibitor. If the substrate concentration is increased, keeping the inhibitor concentration fixed, the degree of inhibition of the enzyme activity decreases.
An opposite effect is observed, if the inhibitor concentration is increased, keeping the substrate concentration fixed. This happens because the substrate and the inhibitor both bind to the same catalytic site of the enzyme by virtue of a similarity in structure of the substrate and the inhibitor. Thus, the substrate and the inhibitor compete with each other for occupying the same active site or sites of an enzyme molecule.
Because of structural similarity, the enzyme molecule cannot distinguish between the correct substrate and the false one which is the inhibitor. An often cited example of competitive inhibition is the inhibition of succinic acid dehydrogenase by malonic acid. In the TCA cycle succinic acid is dehydrogenated by succinic dehydrogenase to fumaric acid, FAD acting as H-acceptor. In presence of malonic acid, the enzyme can combine with the inhibitor, but fails to dehydrogenate it.
The structures of succinic and malonic acids are shown:
Another well-known example of competitive inhibition having clinical importance is that of sulfanilamide and p-amino benzoic acid. Sulfanilamide forms the nucleus of all sulfa-drugs which are used as chemotherapeutic agents against a variety of infections caused by bacteria. Par-amino-benzoic acid (p-ABA) is an essential vitamin required by many bacteria for synthesis of folic acid which acts as a coenzyme.
The enzyme which acts on p-ABA to convert it to the next intermediate in the biosynthesis of folic acid is competitively inhibited by sulfanilamide, because p-ABA has structural similarity with the inhibitor. The bacteria are deprived of folic acid and are unable to grow.
The structures of the two compounds are shown below:
Non-Competitive Inhibition:
A non-competitive inhibition cannot be reversed by increasing substrate concentration, because the inhibitor does not bind to the enzyme protein at the same active site as the normal substrate, but at a different site. Hence there is no competition between the substrate and the inhibitor.
The inhibition may be caused due to a change in the shape of the substrate-site due to binding of the inhibitor to the same enzyme molecule though at a different site. This type of non-competitive inhibition is also known as allosteric inhibition and has been dealt with separately.
The more common type of non-competitive inhibition is that exerted by the heavy metal ions that bind reversibly with the sulfhydryl group (-SH) of cysteine residues of enzyme proteins. For many enzymes, the free -SH groups are essential for catalytic activity, because they are often involved in maintaining the correct three-dimensional configuration of the enzyme protein required for its catalytic function. Heavy metal ions, like Hg++ and Ag++ bind to the -SH groups of enzyme proteins reversibly, causing non-competitive inhibition.
Non-competitive inhibition may also be due to some agents which bind inorganic co-factors required by certain apo-enzymes to form functional holoenzymes. These inorganic co-factors are usually divalent metal ions, like Mg++, Ca++, Fe++ etc. The inhibitors which bind such metal ions include cyanide which binds Fe++ or Fe+++, fluoride which binds Ca++ or Mg++, ethylene diamino tetra acetic acid (EDTA) which binds Mg++ and other divalent metal ions, etc.
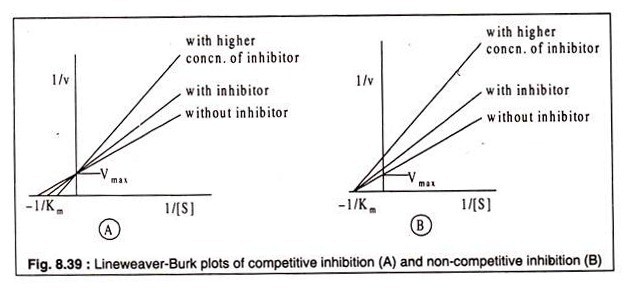
Whether an inhibior acts as a competitive or a non-competitive one can be recognized from their kinetics. Lineweaver-Burk plots using varying concentrations of the inhibitor and a fixed concentration of the substrate reveal the difference between a competitive and a non-competitive inhibitor. In case of competitive inhibition, plots of 1/v against 1/[S] produce straight lines of different slopes, intersecting the 1/v axis at a common point.
The curves indicate that Vmax is not altered by the presence of the inhibitor, but the Km is. In presence of the inhibitor Km has a higher value (Fig. 8.39 A). In case of noncompetitive inhibition, on the other hand, the straight lines also show different slopes with varying concentrations of the inhibitor, but they do not intercept the 1/v axis at the same point as observed in case of competitive inhibition.
Rather the lines meet at a common point on the -1/[S] axis. This indicates that increasing concentration of inhibitor causes decrease in Vmax which is not restored by increasing substrate concentration. The Km, however, remains unchanged, because the substrate and the inhibitor do not bind to the same site of the enzyme (Fig. 8.39B).