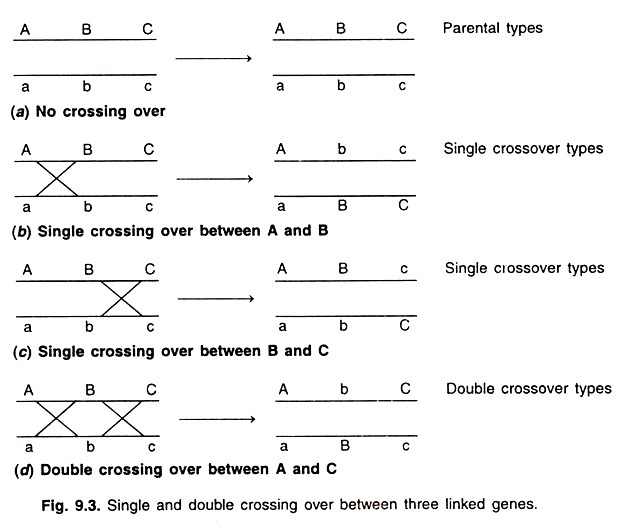
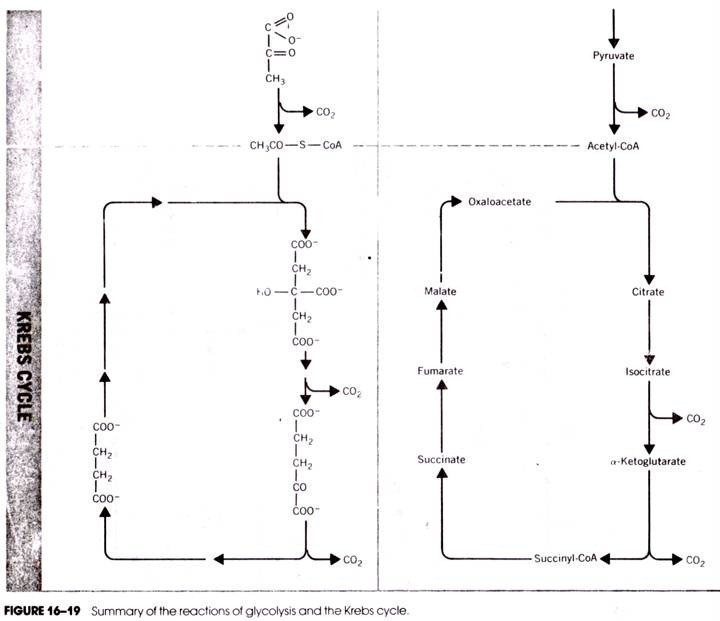
The tricarboxylic acid cycle is also called the Krebs cycle because the major steps of the cycle were worked out in 1937 by Sir Hans Krebs.
At that time, radioactively labeled compounds were not available for biological studies and the cellular site of the reactions was not known with certainty.
The experiments performed by Krebs and the logic and reasoning with which his findings were interpreted in order to formulate the metabolic pathway are recognized as milestones in our progressive unraveling of cellular metabolism.
In 1948 E. P. Kennedy and A. L. Lehninger showed that mitochondria isolated from liver tissue homogenates by differential centrifugation contained the enzyme activities of the Krebs cycle reactions. Krebs received the Nobel Prize in 1953 for his elucidation of this most important metabolic pathway.
The Krebs cycle reactions are utilized by the cell to further metabolize a number of products of other reactions in the cytosol. These can be products as diverse as amino acids, fatty acids, precursors of nucleic acids, and pyruvic acid. However, the greatest metabolic merit of the cycle is its oxidation of pyruvate— the product of carbohydrate metabolism in the cytosol. The oxidation of this compound in the TCA cycle provides the reducing power to make significant amounts of ATP. The reactions are shown in Figure 16-20 and are described below.
Oxidation of Pyruvate:
The oxidation of pyruvate is brought about by a set of reactions catalyzed by the pyruvate dehydrogenase complex. This complex contains three enzymes (E1 pyruvate dehydrogenase; E2, dihydrolipoyl transacetylase; and E3, dihydrolipoyl dehydrogenase) and five coenzymes and is located in the matrix of the mitochondrion. The first step is the decarboxylation of the pyruvate to yield CO2 and an α-hydroxyethyl unit attached through thiamine pyrophosphate (TPP, a coenzyme) to enzyme E1 that is,
The hydroxyethyl group is then dehydrogenated (oxidized) to form an acetyl group, which is transferred to one of the sulfur atoms of lipoic acid, a coenzyme of E2.
The acetyl group is then transferred to coenzyme A, forming acetyl-CoA, which separates from the enzyme complex. One hydrogen from the lipoyl group and one from coenzyme A are transferred to flavin adenine dinucleotide (FAD) (Fig. 3-11% a coenzyme of E3.
The ∆GO0‘ is -33.5 kJ/mole (-8.0 kcal/ mole), indicating that the reaction is highly exergonic, with the- equilibrium lying well to the right. In most animal tissues, the reaction is considered irreversible.
The Krebs cycle Reactions:
The first step of the Krebs cycle is a condensation reaction between acetyl-CoA and oxaloacetate, a product of the cycle itself. The reaction is catalyzed by citrate synthetase, with citrate produced and CoA released for reutilization in another round of the previous reaction.
The ∆G0‘ is -32.2 kJ/mole (-7.7 kcal/mole). This reaction is the “pacemaker” or primary rate-limiting reaction of the cycle. The rate is controlled by the availability of acetyl-CoA and oxaloacetate. Succinyl-CoA, the product of the later step in the cycle, is a competitive inhibitor of this reaction, because it competes with acetyl-CoA for the active site on the enzyme. The enzymatic conversion of citrate to isocitrate is a two-step process in which the intermediate, cisaconitate, remains attached to the enzyme aconitase and therefore is frequently not shown in the Krebs cycle. The equilibrium of this reaction is toward citrate (i.e., ∆G0‘ is 6.7 kJ/mole or 1.59 kcal/mole), but the isocitrate is rapidly oxidized in the next step, thus shifting the direction of the reaction by removal of the product.
The oxidation of isocitrate to α-ketoglutarate is also a two-step process, with the intermediate remaining attached to the enzyme isocitrate dehydrogenase. The first step is an oxidation, two hydrogens being transferred to NAD+. Actually, there are two isocitrate dehydrogenases in the mitochondrial matrix.
One is linked to NAD+ and the other to NADP+ An NADP + -linked isocitrate dehydrogenase is also found in the cytosol. However, it appears that the NAD + – isocitrate dehydrogenase is the most active form in Krebs cycle reactions. This is an allosteric enzyme and is specifically stimulated by ADP.
When large amounts of ATP are consumed in the cell and corresponding quantities of ADP produced the rising ADP level acts as a positive effector for the allosteric enzyme. Alternatively, when the level of ATP rises, the accompanying fall in the level of ADP diminishes the positive effect on the allosteric enzyme. The overall effect of ADP-dependent NAD +-isocitrate dehydrogenase in controlling the level of Krebs cycle activity is considered secondary, however, to the citrate synthetase step.
The second step of the reaction catalyzed by isocitrate dehydrogenase is the decarboxylation of the β-carboxyl group. The ∆G0‘ for the entire reaction is – 20.9 kJ/mole (- 5.0 kcal/mole).
The oxidation of α-ketoglutarate to succinyl-CoA involves an enzyme complex called the α-ketoglutarate dehydrogenase complex. In the sequence of reactions catalyzed by this complex, CO2 is removed by first complexing with thiamine pyrophosphate. During addition of CoA, two hydrogen atoms removed from lipoic acid and coenzyme A reduce NAD+ to NADH + H +. The ∆G0‘ is -33.5 kJ/mole (-8.0 kcal/ mole).
The removal of CoA from succinyl-CoA is coupled to substrate-level phosphorylation of GDP and is catalyzed by the enzyme succinyl-CoA synthetase.
The ∆G0‘ is -2.9 kJ/mole (-0.7 kcal/mole). Phosphate is attached to the enzyme-succinyl-CoA complex before it is transferred to GDP. In Escherichia coli, the phosphate is transferred to ADP; in animal and most plant tissues GTP is formed and then the GTP donates the phosphate to ADP to form ATP.
GTP + ADP-ATP + GDP …(16-11)
Succinic dehydrogenase catalyzes the oxidation of succinate to fumarate. This is the one enzyme of the TCA cycle reactions that has been shown to be attached to the inner surface of the inner membrane although it is easily removed from the membrane. Succinic dehydrogenase employs a flavin adenine dinucleotide (FAD) as a coenzyme, and it is this coenzyme that accepts the two hydrogens removed from the succinate during oxidation.
succinate + FAD → fumarate + FADH2 …(16-12)
The ∆G0‘ is 0.
Fumarate is converted to malate by fumarase. The reaction has a ∆G0‘ of -3.7 kJ/mole (-0.88 kcal/ mole).
Fumarate + HgO →malate …(16-13)
Malate is oxidized by malate dehydrogenase, which is an NAD + -containing enzyme. Although the AG0‘ is 29.7 kJ/mole (7.1 kcal/mole) and indicates that the isolated reaction
malate + NAD+ →oxaloacetate + NADH + H + …(16-14)
is endergonic, the products of the reaction are readily removed in vivo and the reaction is therefore driven in the direction as written. Malate dehydrogenase is found not only in the matrix of mitochondria but also in the cytosol. The oxaloacetate produced by the reaction can serve to initiate the cycle again by combining with acetyl-CoA to form citrate.
Summary of the TCA Cycle:
It is possible to account for all the atoms that pass through the TCA cycle. There are two’ carbons in acetyl-CoA, and during the cycle one is converted to CO2 at the isocitrate dehydrogenase step (16-8) and the other is converted to CO2 at the α-ketoglutarate dehydrogenase step (16-9). Although these are not the same carbon atoms that entered the cycle, a balance is achieved—two carbons enter the cycle and two leave the cycle.
The same accounting of carbons can be made when starting with glucose.
C6H12O6 + 6 O2-6CO2 + 6H2O …(16-15)
This monosaccharide is broken down during glycolysis in the cytosol (Fig. 16-19) to form two molecules of pyruvate. Each pyruvate molecule loses one carbon to CO2 as it enters the Krebs cycle at the pyruvate dehydrogenase step (16-1 and 16-2) and another at each of the two steps described above in the TCA cycle.
An accounting can also be made for the hydrogen atoms during glycolysis and Krebs cycle reactions. One glucose (C6 H12 O6) and 6 H20 contribute a total of 24 hydrogens. During glycolysis, 4 hydrogens are removed at the glyceraldehyde-3-phosphate dehydrogenase step to form 2 NADH+ 2 H+ in the cytosol and the two pyruvates from glucose each yield two more hydrogens (2 NADH + 2 H+) at the pyruvate dehydrogenase step (16-1 and 16-2).
Four more are transferred to NAD+ at the isocitrate dehydrogenase step (16-8), four are transferred to NAD+ at the a-ketoglutarate dehydrogenase step (16- 8), four more are transferred to FAD at the succinate dehydrogenase step (16-12), and finally another four are transferred to NAD+ at the malate dehydrogenase step (16-14). This accounts for all 24 hydrogens. Although these are not the identical hydrogen atoms that were present in the original glucose and water molecules, a numerical balance is nonetheless achieved.
In a similar way, the oxygens also balance. Glucose contributes 6 oxygens and the 6 water molecules added mean that 12 oxygens enter the system. Twelve oxygen molecules are present in the 6 C02 molecules produced. Taken altogether, the overall equation for the respiration of glucose through the Krebs cycle reactions is
The physiological importance of the Krebs cycle reactions is the production of tremendous chemical reducing power in the matrix of the mitochondrion by the accumulation of NADH, H+, and FADH2. These compounds enter the electron transport system reactions and their reducing potential drives the oxidative phosphorylation reactions that produce ATP.