The polysaccharides (or glycans) are composed of long chains of sugars and can be divided into two main functional groups the structural polysaccharides and the nutrient polysaccharides.
The structural polysaccharides serve primarily as extracellular or intercellular supporting elements.
Included in this group are cellulose (found in plant cell walls), mannan (found in yeast cell walls), chitin (in the shells of arthropods and the cell walls of some fungi), hyaluronic acid, keratan sulfate, and chondroitin sulfate (in connective tissue) and the peptidoglycans of bacteria.
The nutrient polysaccharides serve as reserves of mono-saccharides and are in continuous metabolic turnover. Included in this group are starch (plant cells and bacteria), glycogen (animal cells), and paramylum (in certain protozoa). On chemical bases, the polysaccharides can be divided into two broad classes: the homopolysac- charides and the heteropolysaccharides.
In the homopolysaccharides, all the constituent sugars are the same. Included in this class are cellulose, starch, glycogen, and paramylum. In the heteropolysaccharides, the constituent sugars may take different forms; included here are hyaluronic acid, keratan sulfate, and chondroitin sulfate.
Some polysaccharides are un-branched (i.e., linear) chains whose structure may be ribbon like or helical (usually a left-handed spiral). Other polysaccharides are branched and, like many proteins, assume a globular form. A description of some of the more important polysaccharides follows.
Cellulose:
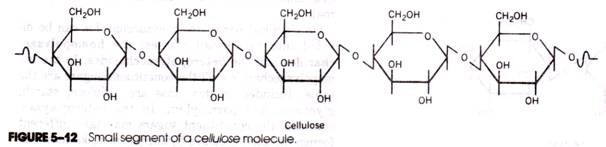
Cellulose is not only the most common of all polysaccharides; it is also the most abundant organic substance in the living world. Indeed, it is estimated that more than half of all organic carbon is present as cellulose. Cellulose is the major component of plant cell walls, where it plays a structural role. Cellulose is an un-branched polymer of glucose in which the neighboring mono-saccharides are joined by β1→4 glycosidic bonds (Fig. 5-12).
Chain lengths vary from several hundred to several thousand glucosyl units (in the algae Valonia, a single molecule of cellulose may contain more than 20,000 glucosyl units). Successive pyranose rings in cellulose are rotated 180° relative to one another so that the chain of sugars takes on a “flip-flop” appearance.
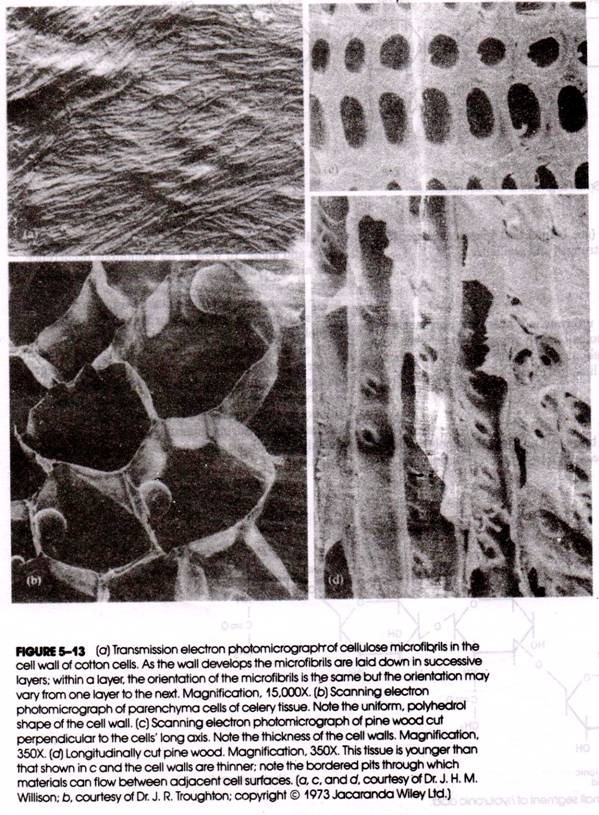
In plant cell walls, large numbers of cellulose molecules are organized into cross-linked, parallel micro fibrils (Fig. 5-13), whose long axis is that of the individual chain. The cellulose micro fibrils may be coated with hemicellulose (a smaller polysaccharide formed from xylose, mannose, or galactose).
The plant cell wall is composed of a series of layers of these micro-fibrils, with the walls of neighboring cells cemented together by pectin (a polymer of galacturonic acid). Vast amounts of cellulose may be deposited between neighboring cells as is- vividly seen in the scanning electron photomicrographs of wood tissue shown in Figure 5-13 Cellulose has also been identified in the cell walls of algae and some fungi.
Chitin:
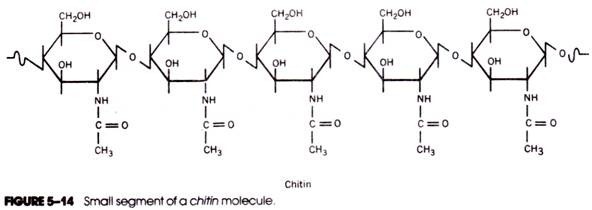
Chitin is an extracellular structural polysaccharide found in large quantities in the body covering (cuticle) of arthropods and in smaller amounts in sponges, mollusks, and annelids. Chitin has also been identified in the cell walls of most fungi and some green algae. The chemical structure of chitin is closely related to that of cellulose; the difference is that the hydroxyl group of each number 2 carbon atom is replaced by an acetamido group. Hence, chitin is an unbranched polymer of N-acetylglucosamine (Fig. 5-14) containing several thousand successive aminosugar units linked by β→4 glycosidic bonds.
Hyaluronic Acid, Keratan Sulfate, and Chondroitin Sulfate:
Cartilage tissue contains the related polysaccharides hyaluronic acid, keratan sulfate, and chondroitin sulfate. Hyaluronic acid is an unbranched heteropoly-saccharide containing repeating disaccharides of N- acetylglucosamine and glucuronic acid (Fig. 5-15). Glucuronic acid is linked to N-acetylglucosamine in each disaccharide by a 1→3 glycosidic bond, but successive disaccharides are 1→4 linked. In addition to cartilage, hyaluronic acid is found in other connective tissues, in the synovial fluid of joints, in the vitreous humor of the eyes, and also in the capsules that enclose bacteria.
Keratan sulfate, like hyaluronic acid, is a repeating disaccharide forming an unbranched chain. Each disaccharide unit of the polysaccharide consists of galactose and sulfated N-acetylglucosamine. Chondroitin sulfate is a repeating disaccharide consisting of alternating glucuronic acid and sulfated N- acetylgalactosamine residues. Hyaluronic acid, keratan sulfate, and chondroitin sulfate, together with a number of oligosaccharides and proteins, form the proteoglycan (see below) that gives cartilage its unusual tensile strength and resilience.
Inulln:
Inulin is an unbranched nutrient polysaccharide found in the bulbs of such plants as artichokes, dahlias, and dandelions. It consists of repeating fructose units in β2 → 1 linkage (Fig. 5-16).
Glycogen:
Glycogen is a branched nutrient homopolysaccharide containing glucose in α1→4 and α1→6 linkages. It is found in nearly all animal cells and also in certain protozoa and algae. In view of its ubiquitous occurrence, it is an extremely important polysaccharide and has been the subject of numerous studies. In man and other vertebrates, glycogen is stored primarily in the liver and muscles and is the principal form of stored carbohydrate. In an unstarved animal, as much as 10% of the liver weight may be glycogen.
Glycogen undergoes almost continuous biosynthesis and degradation, especially in liver tissue. Liver glycogen serves as a reservoir for glucose under starvation conditions (it may be almost completely depleted during 24 hours of fasting) and during muscular exertion. Glycogen is quickly resynthesized from newly ingested carbohydrate.
Glycogen molecules exist in a continuous spectrum of sizes, with the largest molecules containing many thousands of glucosyl units. A small portion of a glycogen molecule is shown in Figure 5-17. The glucosyl units that are linked by α1 → 4 glycosidic bonds are organized into long chains; the chains are interconnected at branch points by α1→6 glycosidic bonds.
This yields the “bush”- or “tree”-like structure depicted in Figure 5-18. It should be noted that in a glycogen molecule there is only one glucose unit whose number 1 carbon atom bears a hydroxyl group. All of the other 1-OH groups are involved in α1 → 4 and α1 → 6 glycosidic bonds. The single free 1-OH group is called the “reducing end” of the molecule and is noted by the letter R in Figure 5-18. In contrast, numerous “non-reducing ends” are present (i.e., free 4-OH and 6-OH groups) at the terminals of the outermost chains.
In Figure 5-18 the individual glucose units are represented by circles and the branch points (i.e., the α1→6 linkages) by heavier connections. In this model of glycogen, a number of different kinds of chains may be distinguished. A chains are attached to the molecules by a single 1 → 6 linkage (chains of open circles in Fig. 5-18) and B chains (gray circles) bear one or more A chains. Each glycogen molecule contains only one C chain (colored circles), and this is the chain that ends in the free reducing group.
Exterior chains are those portions of individual chains between the non-reducing end groups and the outermost branch points. Finally, those parts of individual chains between branch points are called interior ‘chains. The exterior chains of glycogen are usually six to nine glucosyl units long, whereas interior chains contain only three to four glucosyl units. Approximately 8 to 10% of all glycosidic linkages are the 1→6 type.
Glycogen molecules are often sufficiently large to be studied by electron microscopy. Although the molecules can be seen in transmission electron photomicrographs of osmium tetroxide-fixed tissues , morphological studies of glycogen are usually carried out with material that has been negatively stained with phosphotungstic acid and osmium tetroxide (Fig. 5-19).
Starch:
Starch is a nutrient polysaccharide found in plant cells, protists, and certain bacteria and is similar in many respects to glycogen. (In fact, glycogen is often referred to as “animal starch.”) Starch usually occurs in cells in the form of granules visible by both light and electron microscopy.
In plant cells (such as potato or corn) these granules may be several micrometers in diameter and may account for more than half of the cell’s dry weight. In microorganisms the starch granules are smaller, having diameters of only 0.5 to 2 μm. Starch granules contain a mixture of two different polysaccharides, amylose and amylopectin, and the relative amounts of these two polysaccharides vary according-to the source of the starch.
The amyloje component of starch is an unbranched 1→4 polymer of glucose and may be several thousand glucosyl units long. The polysaccharide chain exists in the form of a left-handed helix containing six glucosyl residues per turn (Fig. 5-20). The familiar blue color that is produced when starch is treated with iodine is believed to result from the coordination of iodine ions in the interior of the helix. The polysaccharide must contain at least six helical turns (i.e., 36 glucosyl residues) to produce the characteristic blue color when treated with iodine.
Amylopectin is a branched polysaccharide containing 1→4 and 1→6 linked glucosyl units; in this respect it is similar to glycogen. However, amylopectin has a more open structure with fewer 1→6 linkages and longer chain lengths. Some of the characteristics of amylopectin and glycogen are compared in Table 5-1.
Other Polysaccharides:
In addition to the polysaccharides already described, several others should be briefly reviewed. Mannan, a homopolymer of mannose, is found in the cell walls of yeast and is also stored intracellularly in some plants. In yeast, the mannan has a branched structure, whereas in plants it is a linear molecule.
Paramylum is a nutrient homopolysaccharlde stored intracellularly as large granules in certain protozoa (e.g., Euglena). It consists of unbranched chains of glucosyl units in 1→3 linkage. Dextran is a branched homo- polymer of glucose produced by certain bacteria.However, unlike glycogen and amylopectin, the glycosidic linkages vary from one species to another and maybe 1→6, 1→4, 1→3, or 1→2. Some of the properties of the various polysaccharides are summarized in Table 5-2.