The pharmaceutical products of recombinant DNA technology are broadly divided into the following three categories and briefly discussed with important examples:
1. Human protein replacements.
2. Therapeutic agents for human diseases.
3. Vaccines.
Some authors do not make such categorization and consider all of them together as pharmaceutically important products of biotechnology.
Contents
Type # 1. Human Protein Replacements:
The synthesis of the cellular proteins is ultimately under the control of genes. Any defect in a gene produces an incorrect protein or no protein at all. Sometimes, the occurrence of a defective (i.e. functionally ineffective) or deficient protein may cause a disease. Thus, gene defects will result in inherited or genetically linked diseases.
Identification of defective or deficient proteins in the causation of inherited diseases is very important. The recombinant DNA technology can be fruitfully employed to produce human proteins that can be used for the treatment of genetically linked diseases. This is referred to as human protein replacement strategy in biotechnology.
Insulin:
The hormone insulin is produced by the (J-cells of islets of Langerhans of pancreas. Human insulin contains 51 amino acids, arranged in two polypeptide chains. The chain A has 21 amino acids while B has 30 amino acids. Both are held together by disulfide bonds.
Diabetes mellitus:
Diabetes mellitus affects about 2-3% of the general population. It is a genetically linked disease characterized by increased blood glucose concentration (hyperglycemia). The occurrence of diabetes is due to insufficient or inefficient (incompetent) insulin. Insulin facilitates the cellular uptake and utilization of glucose for the release of energy.
In the absence of insulin, glucose accumulates in the blood stream at higher concentration, usually when the blood glucose concentration exceeds about 180 mg/dl, glucose is excreted into urine. The patients of diabetes are weak and tired since the production of energy (i.e. ATP) is very much depressed.
The more serious complications of uncontrolled diabetes include kidney damage (nephropathy), eye damage (retinopathy), nerve diseases (neuropathy) and circulatory diseases (atherosclerosis, stroke). In fact, diabetes is the third leading cause of death (after heart disease and cancer) in many developed countries.
In the early years, insulin isolated and purified from the pancreases of pigs and cows was used for the treatment of diabetics. There is a slight difference (by one to three amino acids) in the structure of animal insulin compared to human insulin. This resulted in allergy in some of the diabetics when animal insulin was administered.
Another problem with animal insulin is that large number of animals have to be sacrificed for extracting insulin from their pancreases. For instance, about 70 pigs (giving about 5 kg pancreatic tissue) have to be killed to get insulin for treating a single diabetic patient just for one year!
Production of recombinant insulin:
Attempts to produce insulin by recombinant DNA technology started in late 1970s. The basic technique consisted of inserting human insulin gene and the promoter gene of lac operon on to the plasmids of E. coli. By this method human insulin was produced. It was in July 1980, seventeen human volunteers were, for the first time, administered recombinant insulin for treatment of diabetes at Guy’s Hospital, London.
And in fact, insulin was the first ever pharmaceutical product of recombinant DNA technology administered to humans. Recombinant insulin worked well, and this gave hope to scientists that DNA technology could be successfully employed to produce substances of medical and commercial importance. An approval, by the concerned authorities, for using recombinant insulin for the treatment of diabetes mellitus was given in 1982. And in 1986, Eli Lilly Company received approval to market human insulin under the trade name Humulin.
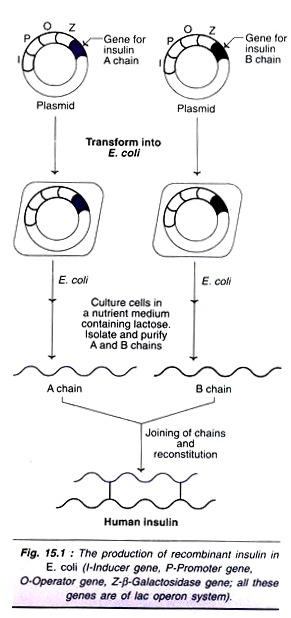
Technique for recombinant insulin production:
The original technique of insulin synthesis in E. coli has undergone several changes, for improving the yield, e.g. addition of signal peptide, synthesis of A and B chains separately etc. The procedure employed for the synthesis of two insulin chains A and B is illustrated in Fig. 15.1. The genes for insulin A chain and B chain are separately inserted to the plasmids of two different E. coli cultures.
The lac operon system (consisting of inducer gene, promoter gene, operator gene and structural gene Z for β-galactosidase) is used for expression of both the genes. The presence of lactose in the culture medium induces the synthesis of insulin A and B chains in separate cultures. The so formed insulin chains can be isolated, purified and joined together to give a full-pledged human insulin.
Second generation recombinant insulin’s:
After injecting the insulin, the plasma concentration of insulin rises slowly. And for this reason, insulin injection has to be done at last 15 minutes before a meal. Further, decrease in the insulin level is also slow, exposing the patients to a danger of hyper-insulinemia. All this is due to the existence of therapeutic insulin as a hexamer (six molecules associated), which dissociates slowly to the biologically active dimer or monomer.
Attempts have been made in recent years to produce second generation insulin by site- directed mutagenesis and protein engineering. The second generation recombinant proteins are termed as muteins. A large number of insulin muteins have been constructed with an objective of faster dissociation of hexamers to biologically active forms. Among these is insulin lispro, with modified amino acid residues of the B-chain of insulin. Insulin lispro can be injected immediately before a meal as it attains the pharmacologically efficient levels very fast.
Chemically altered porcin insulin:
As already stated, porcin (pig) insulin differs from human insulin just by one amino acid-alanine in place of threonine at the C-terminal and of B-chain of human insulin. Biotechnologists have developed methods to alter the chemical structure of porcin insulin to make it identical to human insulin. And this chemically modified porcin insulin can also be employed for the treatment diabetes mellitus.
Human Growth Hormone:
Growth hormone is produced by the pituitary gland. It regulates the growth and development. Growth hormone stimulates overall body growth by increasing the cellular uptake of amino acids, and protein synthesis, and promoting the use of fat as body fuel.
Insufficient human growth hormone (hGH) in young children results in retarded growth, clinically referred to as pituitary dwarfism. The child usually is less than four feet in height, and has chubby face and abundant fat around the waist.
Traditional treatment for dwarfism:
The children of pituitary dwarfism were treated with regular injections of growth hormone extracted from the brains of deceased humans. It may be noted that only human growth hormone is effective for treatment of dwarfism. (This is in contrast to diabetes where animal insulin’s are employed).
At least eight pituitary glands from cadavers must be extracted to get hGH adequate for treating a dwarf child just for one year! And such treatment has to be continued for 8-10 years!! Further, administration hGH isolated from human brains exposes the children to a great risk of transmitting the cadaver brain diseases (through virus or viral-like agents) e.g. Creuzfeldt- Jacob (CJ) syndrome characterized by convulsions, wasting of muscle etc.
Production of recombinant hGH:
Biotechnologists can now produce hGH by genetic engineering. The technique adopted is quite comparable with that of insulin production. The procedure essentially consists of inserting hGH gene into E. coli plasmid, culturing the cells and isolation of the hGH from the extracellular medium.
Limitation in hGH production:
The hGH is a protein comprised of 191 amino acids. During the course of its natural synthesis in the body, hGH is tagged with a single peptide (with 26 amino acids). The signal peptide is removed during secretion to release the active hGH for biological functions. The entire process of hGH synthesis goes on in an orderly fashion in the body.
However, signal peptide interrupts hGH production by recombinant technology. The complementary DNA (cDNA) synthesized from the mRNA encoding hGH is inserted into the plasmid. The plasmid containing E. coli when cultured, produces full length hGH along with signal peptide. But E. coli cannot remove the signal peptide.
Further, it is also quite difficult to get rid of signal peptide by various other means. Theoretically, cDNA encoding signal peptide can be cut to solve these problems. Unfortunately, there is no restriction endonuclease to do this job, hence this is not possible.
A novel approach for hGH production:
Biotechnologists have resolved the problem of signal peptide interruption by a novel approach (Fig. 15.2). The base sequence in cDNA encoding signal peptide (26 amino acids) plus the neighbouring 24 amino acids (i.e a. total 50 amino acids) is cut by restriction endonuclease ECoRI.
Now a gene (cDNA) for 24 amino acid sequence of hGH (that has been deleted) is freshly synthesized and ligated to the remaining hGH cDNA. The so constituted cDNA, attached to a vector, is inserted into a bacterium such as E. coli for culture and production of hGH. In this manner, the biologically functional hGH can be produced by DNA technology. Recombinant hGH was approved for human use in 1985. It is marketed as Protropin by Gene-tech Company and Humatrope by Eri Lilly Company.
Controversy over the use of hGH:
Recombinant hGH can be administered to children of very short stature. It has to be given daily for many years with an annual cost of about $ 20,000. Some workers have reported substantial increase in the height of growth retarded children.
One group of workers observed that the normal growth pattern in children was not restored on hGH administration, although there was an initial spurt. Another big question raised by the opponents of hGH therapy is whether it is necessary to consider short stature as a disorder at all for treatment!
Use of recombinant growth hormone for farm animals:
Recombinant GH is now available for administration to farm animals to promote early growth and development. Such farm animals yield linear meat, besides increased milk production. However, use of GH in farm animals is a controversial issue.
Clotting Factor VIII:
The clotting factor VIII is required for proper blood clotting process. A genetic defect in the synthesis of factor VIII results in the disorder hemophilia A. This is a sex-linked disease (incidence 1 in 10,000 males) transmitted by females affecting males. The victims have prolonged clotting time and suffer from internal bleeding.
Traditional treatment for hemophilia A:
Clotting factor VIII was isolated from the whole blood and administered to the patients of hemophilia A. This approach requires large quantities of blood. Another problem is the risk of transmission of certain diseases like AIDS to the hemophiliacs.
Production of recombinant factor VIII:
The gene for the formation of factor VIII is located on X chromosome. It is a complex gene of 186 kb (i.e., 186,000 base pairs) in size, organized into 26 exons of varying length. In between the exons, many introns are present. The introns vary in their size, starting from 200 base pairs to as high as 32,000 base pairs.
Biotechnologists were able to isolate mature mRNA (containing only exons and no introns) that is responsible for the synthesis of factor VIII. This mRNA contains 9,000 bases and synthesizes the protein, factor VIII. Factor VIII contains 2332 amino acids, with carbohydrate molecules attached at least at 25 sites.
DNA technologists synthesized the complementary DNA (cDNA) for mature mRNA of factor VIII. This cDNA can be inserted into mammalian cells or hamster kidney cells for the production of recombinant factor VIII. Since 1992, factor VIII is available in the market. It is produced by Genetics Institute in Cambridge, Massachusetts and sold as Recombinant while Miles Laboratories sell under the trade name Kogenate.
Type # 2. Therapeutic Agents for Human Diseases:
Biotechnology is very useful for the production of several therapeutic products for treating human diseases. A selected list of rDNA-derived therapeutic agents along with trade names and their uses in humans are given in Table 15.3.
Some of these are described above (under human protein replacements) while the remaining are discussed below:
Tissue Plasminogen Activator:
Tissue plasminogen activator (tPA) is a naturally occurring protease enzyme that helps to dissolve blood clots. tPA is a boon for patients suffering from thrombosis. The majority of natural deaths worldwide are due to a blockade of cerebral or coronary artery by a blood clot, technically called as thrombus. The phenomenon of thrombus blockage of blood vessels is referred to as thrombosis.
Chemically, thrombus consists of a network of fibrin, formed from the fibrinogen. In the normal circumstances, plasmin degrades fibrin and dissolves blood clots. This plasmin is actually produced by activation of plasminogen by tissue plasminogen activator (Fig. 15.3).
The natural biological systems is however, not that efficient to remove the blood clots through this machinery. Tissue plasminogen activator is very useful as a therapeutic agent in dissolving blood clots (thrombi) by activating plasminogen. By removing the arterial, thrombi, the possible damage caused by them on heart and brain could be reduced.
Production of recombinant tPA:
DNA technologists synthesized the complementary DNA (cDNA) molecule for tissue plasminogen activator. This cDNA was then attached to a synthetic plasmid and introduced into mammalian cells (Fig. 15.4). They were cultured and tPA-producing cells were selected by using methotrexate to the medium.
tPA-producing cells were transferred to an industrial tank (fermenter). tPA, secreted into the culture medium, is isolated for therapeutic purpose. It may be noted here that tPA was the first pharmaceutical product to be produced by mammalian cell culture.
Recombinant tPA has been in use since 1987 for treatment of patients with acute myocardial infarction or stroke. Gene-tech was the first to market tPA with a trade name Activase.
Alteplase and Reteplase:
These are the second generation recombinant tPAs. They have increased in vivo half-lives and are functionally more efficient. The general aspects of second generation recombinant proteins are given elsewhere.
Antibody-plasminogen activator conjugates:
An antibody against fibrin (anti-fibrin antibody) can be conjugated with tissue plasminogen activator. This conjugate is appropriately regarded as immunotherapeutic thrombolytic agent. It quickly and specifically binds to fibrin clots and locally increases the conversion of plasminogen to plasmin to dissolve fibrin (Fig. 15.5). In fact, anti-fibrin monoclonal antibodies have been synthesized, conjugated with tPA and tried for solubilizing blood clots.
Advantages of tPA as thrombolytic agent:
Tissue plasminogen activator acts on blood clots (solubilizes by degradation) without reducing the blood clotting capability elsewhere. This is in contrast to the action of urokinase or streptokinase which are more generalized in their action.
Further, tPA can be administered intravenously while urokinase and streptokinase have to be administered directly to the blocked blood vessel. Another merit of using tPA is that its action is much faster than other thrombolytic agents with much reduced side effects.
Interferons:
Interferon is an antiviral substance, and is the first line of defense against viral attacks. The term interferon has originated from the interference of this molecule on virus replication. It was originally discovered in 1957 by Alick Isaacs and Jean Lindemann and was considered to be a single substance.
It is now known that interferon actually consists of a group of more than twenty substances with molecular weights between 20,000-30,000 daltons. All the interferons are proteins in nature and many of them are glycoproteins. They are broadly categorized into three groups based on their structure and function
Interferon-α (IFN-α)
Interferon-β (IFN-β)
Interferon-ƴ (IFN-ƴ)
Mechanism of action of interferons:
Interferons are produced by mammalian cells when infected by viruses. As the virus releases its nucleic acid into cellular cytoplasm, it stimulates the host DNA to produce interferons. These interferons, secreted by the cells, bind to the adjacent cells. Here, they stimulate the cellular DNA to produce a series of antiviral enzymes.
The so formed proteins inhibit viral replication and protect the cells (Fig. 15.6). It is believed that the protective (enzymes) proteins bind to mRNA of viruses and block their protein synthesis. The action of interferons appears to be species specific. Thus, human interferons operate in humans. Other animal (dog, mouse) interferons are ineffective in man.
Isolation of interferons in the early years:
Blood was the only source of interferons earlier. The procedure was very tedious and the quantity of interferons isolated was very little. Thus, as much as 50,000 litres of human blood was required to get just 100 mg of interferons. Therefore, it was very difficult to conduct research or use interferons for therapeutic purposes.
Production of recombinant interferons:
The complementary DNA (cDNA) was synthesized from the mRNA of a specific interferon. This is inserted to a vector (say plasmid) which is introduced into E. coli or other cells. The interferon can be isolated from the culture medium. This is the basic mechanism of producing recombinant interferons.
The production of interferons is relatively less in bacterial hosts, although E. coli was the first to be used. This is mainly because most interferons are glycoproteins in nature and bacteria do not possess the machinery for glycosylation of proteins.
Production interferons by yeasts:
The yeast Saccharomyces cerevisiae is more suitable for the production of recombinant interferons. This is mainly because the yeast possess the mechanism to carry out glycosylation of proteins, similar to that occurs in mammalian cells. The DNA sequence coding for specific human interferon can be attached to the yeast alcohol dehydrogenase gene in a plasmid and introduced into 4 yeast cells. The yield of interferons is several fold higher compared to E. coli.
Production of hybrid interferons:
Several attempts have been made to produce hybrid interferons. This is advantageous since different interferons with different antiviral activities can be combined to produce a more efficient interferon. Further, the glycosylation step can be bypassed, and bacteria can be used to produce hybrid interferons. The hybrid interferons are more reactive in performing their function.
The creation of hybrid genes from the genes of IFN-α2 and IFN-α3 is illustrated in Fig. 15.7. These genes are digested by restriction endonucleases. The resulting fragments are ligated to generate hybrid genes. The appropriate hybrid genes can be selected and used for producing hybrid interferons. E. coli can be employed for this purpose.
Therapeutic applications of interferons:
Interferons-α, -β and -ƴ were respectively approved for therapeutic use in humans in the years 1986, 1993 and 1990. A Swiss biotechnology firm was the first to market interferon-α with a trade name Intron. Interferons are used for the treatment of a large number of viral diseases and cancers.
The cancers include leukemia, kaposis sarcoma, bladder cancer, head and neck cancer, renal cell carcinoma, skin cancer and multiple myeloma. The other diseases employing interferon therapy are AIDS, multiple sclerosis, genital warts, hepatitis C, herpes zoster etc.
Interferons are also employed in the treatment of common colds and influenza. For this purpose, interferons can be used as nasal sprays. The basic mechanism of action of interferons against viruses has already been described.
Interferons are found to cause the death of cancerous cells. This is brought out by stimulating the action of natural killer (NK) cells, a specialised form of lymphocytes that can destroy cancerous cells. Despite the widespread therapeutic applications of interferons, they are not within the reach of a large number of common people due to the cost factor (the cost of production being very high).
Erythropoietin:
Erythropoietin is a hormone synthesized by the kidneys. It stimulates the stem cells of bone marrow to produce mature erythrocytes. Biotechnologists were successful in producing recombinant erythropoietin. An approval for its therapeutic use in humans was obtained in the year 1989. Amgen Inc. first marked erythropoietin with a trade name Epogen. It is useful in treating the patients with severe anemia that accompanies kidney disease.
Another firm Ortho-Biotech company produced Procrit, a genetically engineered erythropoietin in 1997. Procrit acts like the natural hormone and stimulates the production of erythrocytes. It is used in anemic patients undergoing non-cardiac, nonvascular surgery. Procrit administration before surgery serves as an alternative to blood transfusion. However, therapeutic use of procrit is quite expensive, hence not widely used.
Deoxyribonuclease I (DNase I):
The enzyme DNase I hydrolysis long DNA chains into shorter oligonucleotides. The biotechnology firm Genentech isolated and expressed the gene to produce recombinant DNase I. This enzyme is very useful in the treatment of common hereditary disease cystic fibrosis, as explained hereunder.
Cystic fibrosis (CF) is one of the most common (frequency 1: 25,000) genetic diseases. Patients of CF are highly susceptible to lung infections by bacteria. The presence of live or dead bacteria leads to the accumulation of thick mucus in the lungs making the breathing very difficult.
The major constituent of this mucus is the bacterial DNA (released on bacterial lysis). Administration of the enzyme DNase I to the lungs of CF patients decreases the viscosity of the mucus, and the breathing is made easier. It must be remembered that DNase I cannot cure cystic fibrosis. It can only relieve the severe symptoms of the disease in most patients.
Alginate Lyase:
Alginate lyase acts on a polysaccharide polymer namely alginate. Alginate is found in soil and marine bacteria. The occurrence of mucus in the lungs of cystic fibrosis patients is partly due to alginate, produced by the bacterium Pseudomonas aeruginosa. Therefore, administration of alginate lyase instead of or in addition to DNase I helps to clear lungs of CF patients.
Alginate lyase gene has been isolated from a Gram-negative soil bacterium, Flavobacterium sp. This gene was used to produce recombinant alginate lyase in E. coli. Trails are being conducted for therapeutic use of this enzyme in CF patients.
Second Generation Therapeutic Proteins (Muteins):
By employing site-directed mutagenesis, the amino acid sequence of a recombinant protein can be suitably modified as desired, by a technique referred to as protein engineering. The mutated proteins are collectively referred to as muteins.
Protein engineering is a rational approach to modify a protein with regard to its stability, solubility, specificity, substrate affinity, pharmacokinetics etc. The muteins obtained by protein engineering technique are considered as Second generation of therapeutic proteins. Selected examples of such proteins (e.g. insulin lispro, Alteplase) are already described.
Type # 3. Vaccines:
Vaccines are another important group of pharmaceutical products of recombinant DNA technology.