In this article we will discuss about the Structure and Functions of DNA.
Structure of DNA:
DNA or deoxyribose nucleic acid is a helically twisted double chain polydeoxyribonucleotide macromolecule which constitutes the genetic material of all organisms with the exception of riboviruses.
In prokaryotes it occurs in nucleoid and plasmids. This DNA is usually circular. In eukaryotes, most of the DNA is found in chromatin of nucleus. It is linear. Smaller quantities of DNA are found in mitochondria and plastids (organelle DNA).
It may be circular or linear. Single-stranded DNA occurs as a genetic material in some viruses (e.g., coli-phage ф x 174). DNA is the largest macromolecule with a diameter of 2 nm (20A) and having a length in millimeters.
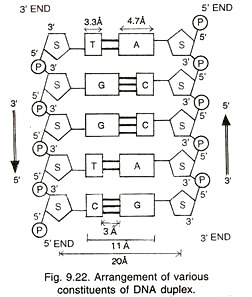
It is a long chain polymer of several hundred thousands of deoxyribonucleotides, e.g., 4.7 million base pairs in E.coli and more than 3 billion base pairs in human being. A DNA molecule has two un-branched complementary strands. They are spirally coiled. The two spiral strands of DNA are collectively called DNA duplex (Fig. 9.21).
The two strands are not coiled upon each other but double strand is coiled upon itself around a common axis like a rope or spiral stair case with base pairs forming steps (rungs) while the back bones of the two strands form railings.
Due to spiral twisting, the DNA duplex comes to have two types of alternate grooves, major and minor. One turn of 360° of the spiral has about 10 nucleotides on each strand of DNA. It occupies a distance of about 3.4 nm (34A) i.e., pitch of DNA is 34A so that adjacent nucleotides or their bases are separated by a space of less than 0.34 nm (3.4 A).
A deoxyribonucleotide of DNA is formed by cross- linking of three chemicals—phosphoric acid (H3PO4), deoxyribose sugar (C5H10O4) and a nitrogen base. Four types of nitrogen bases occur in DNA.
They belong to two groups, purines (9-membered double rings with nitrogen at 1, 3, 7 and 9 positions) and pyrimidine’s (six membered rings with nitrogen at 1 and 3 positions). DNA has two types of purines (adenine or A and guanine or G) and two types of pyrimidine’s (cytosine or С and thymine or T).
Depending upon the type of nitrogen base, DNA has four kinds of deoxyribonucleotides —deoxy-adenosine 5-mono- phosphate (d AMP), deoxy guaninosine 5-monophosphate (d GMP), deoxy thymidine 5-monophosphate (d TMP) and deoxy cytidine 5-monophosphate (d CMP).
The back bone of a DNA chain or strand is built up of alternate deoxyribose and phosphoric acid groups. The phosphate group is connected to carbon 5′ of the sugar residue of its own nucleotide and carbon 3′ of the sugar residue of the next nucleotide by phosphodiester bonds. -H of phosphate and -OH of sugar are eliminated as H2O during each ester formation.
At one end of DNA strand, last sugar has its 5-C free while at other end 3-C of first sugar is free. They are respectively called 5′ and 3′ ends. Phosphate group provides acidity to the nucleic acids because at least one of its side group is free to dissociate.
Nitrogen bases lie at right angles to the longitudinal axis of DNA chains. They are attached to carbon atom 1 ‘of the sugars by glycosidic bonds. Pyrimidine is attached to deoxyribose by its N-atom at 1′ position while a purine does so by N-atom at 9’ position.
The two DNA chains are antiparallel that is, they run parallel but in opposite directions. In one chain the direction is 5′ → 3′ while in the opposite one it is 3′ → 5′ (Fig. 9.22). The two chains are held together by hydrogen bonds between their bases. Adenine (A), a purine of one chain lies exactly opposite thymine (T), a pyramidine of the other chain. Similarly, cytosine (C, a pyramidine) lies opposite guanine (G, a purine).
This allows a sort of lock and key arrangement between large sized purine and small sized pyrimidine. It is strengthened by the appearance of hydrogen bonds between the two. Three hydrogen bonds occur between cytosine and guanine (CsG) at positions 1-1′, 2′ – 6′ and 6′ -2’.
There are two such hydrogen bonds between adenine and thymine (A=T) which are formed at positions 1’ -3′ and 6′ -4’. Hydrogen bonds occur between hydrogen of one base and oxygen or nitrogen of the other base. Since specific and different nitrogen bases occur on the two DNA chains, the latter are complementary.
Thus the sequence of say AAGCTCAG of one chain would have a complementary sequence of TTCGAGTC on the other chain. In other words, the two DNA chains are not identical. It is because of specific base pairing with a purine lying opposite a pyrimidine. This makes the two chains 2 nm thick.
A purine-purine base pair will make it thicker while a pyrimidine- pyrimidine base pair will make it narrower than 2 nm. A larger sized purine, therefore, lies opposite the smaller-sized pyrimidine, A opposite T and С opposite G. This specific base pairing makes the two chains complementary.
Sense and Antisense Strands:
Both the strands of DNA do not take part in controlling heredity and metabolism. Only one of them does so. The DNA strand which functions as template for RNA synthesis is known as template strand, minus (-) strand or antisense strand.
Its complementary strand is named non-template strand, plus (+) strand, sense or coding strand. The latter name is given because by convention DNA genetic code is written according to its sequence.
(5′) GCATTCGGCTAGTAAC (3′)
DNA Non-template, Sense (+) or Coding Strand
(3′) CGTAAGCCGATCATTG (5′)
DNA Template, Antisense, or Noncoding or (-) Strand
(5′) G С AU U С G G С U AG U A AC (3′)
RNA Transcript
RNA is transcribed on 3′ → 5′ (-) stranu (template/anti-strand) of DNA in 5 ←3 direction. The (+) strand of DNA is that coding strand which carries genetic information but is non-template. The term antisense is also used in wider prospective for any sequence or strand of DNA (or RNA) which is complementary to mRNA.
Denaturation (= Melting):
The hydrogen bonds between the nitrogen bases of complementary DNA strands can break due to high temperature, low or high pH. The phenomenon is called denaturation or melting. Since an A—T base pair has only two hydrogen bonds, the area rich in A—T base pairs can undergo easy denaturation.
It is known as low melting area. The area rich in G—С base pairs is comparatively more stable because three hydrogen bonds connect the complementary nitrogen bases. DNA strands separated by melting can re-associate and form duplex. The phenomenon is called renaturation.
Palindromic and Repetitive DNA:
DNA duplex possesses areas where sequence of nucleotides is the same but opposite in the two strands, e.g.,
—T—T—A—A—С—G—T—T—A—A—
—A—A—T—T—G—С—A—A—T—T
These areas are called palindromes or palindromic regions. Regions connected with transcription of ribosomal RNA are often palindromic. The exact significance of this sort of arrangement is not known.
Functions of DNA:
(1) DNA is genetic material which carries all the hereditary information coded in the arrangement of its nitrogen bases.
(2) It has the property of replication (autocatalytic function) essential for passing genetic information from one cell to its daughters or from one generation to the next.
(3) Crossing over produces re-combinations.
(4) Changes in sequence and number of nucleotides produce mutations. Mutations are the fountain head of all variations and formation of new species.
(5) It gives rise to RNAs through transcription (Heterocatalytic function).
(6) DNA controls the metabolic reactions of cells through RNAs and RNA-directed synthesis of proteins, enzymes and other bio-chemicals.
(7) Differentiation of various body parts is due to differential functioning of specific parts of DNA.
(8) Developmental stages occur in the life cycle of an organism by an internal clock of DNA functioning.