Kinds of Chromosomes: Lampbrush, Polytene and Supernumerary!
[I] Lampbrush chromosomes:
These are the largest known chromosomes found in the yolk rich oocytic nuclei of certain vertebrates such as fishes, amphibians, reptiles and birds.
They can be seen with naked eye and are characterized by fine lateral loops, arising from the chromomeres, during first prophase (diplotene) of meiosis.
These loops give it a brush-like appearance; that is why these are called lampbrush chromosomes first discovered by Flemming in 1882 and were described in shark oocytes by Ruckert (1892). Lampbrush chromosomes of certain urodele oocytes may reach upto 5900µ in length.
It consists of longitudinal axis formed by a single DNA molecule along which several hundred bead-like chromomeres are distributed in a linear fashion. From each chromomere there emerge two symmetrical lateral loops (one for each chromatid), which are able to expand or contract in response to various environmental conditions.
About 5 to 10% of the DNA is in the lateral loops. Loop formation reduces the mass of the corresponding chromomeres, implying a spinning out of chromomere material into the lateral strands. The centromeres also have the appearance of elongate Feulgen-positive chromomeres but they characteristically lack lateral loops.
Lampbrush chromosomes can be dissected in (toto) from oocyte nucleus. Individual chromosomes are liable to stretching. With extreme stretching, chromomeres begin to separate transversely into two halves, so that the paired loops form double stranded bridges. The axis between chromomeres is also double, which can be seen in certain special regions where two elements separate longitudinally and bear single loops (Callan, 1955).
These experiments indicate that each chromomere possesses four quadrants separated by both a transverse and a longitudinal line of division (Fig. C). Callan (1963) regards it as that the entire chromatid pair is made up of two continuous strands, which lie parallel to one another in the interchromomere regions, are tightly folded in the chromomeres, and separate as single, unfolded fibres in the loops. Each of the two fibres would correspond to one conventional metaphase chromatid.
There is fundamental similarity in the organization of amphibian lmpbrush chromosomes and dipteran giant polytene chromosomes: in both cases, very long single fibres correspond to single chromatids and are partly but not completely extended. The substructure of the salivary chromosome ‘puffs’ also bear some similarity to that of the lampbrush lateral loops.
Lateral loops are formed of DNA, in chromomeres regions DNA is tightly folded and transcriptionally inactive. In lateral loops RNA synthesis is intense. Each loop in turn has an axis formed by a single DNA molecule, which is coated by a matrix of nascent RNA and proteins. The matrix is asymmetrical, being thicker at one end of the loop. RNA synthesis starts at the thinner end and progresses toward the thicker end.
1. Functions of Lampbrush chromosomes,
(a) Synthesis of RNA:
Functions of lampbrush chromosomes involve synthesis of RNA and protein by their loops. RNA is synthesized only at the thin insertion and then carried around the loops to the thick insertion. There it may be either destroyed or released into nucleus.
(b) Formation of yolk material:
There are some probabilities that lampbrush chromosomes help in the formation of certain amount of yolk material for the egg.
[II] Polytene chromosomes:
These are also giant chromosomes but relatively smaller than lampbrush chromosomes, found in the larvae of certain dipterans. Such banded chromosomes occur in the larval salivary glands, midgut epithelium, and rectum and Malpighian tubules of various genera (Drosophila, Sciara, Rhynchosciara, and Chironomus). In these larvae the salivary glands contain salivary cells so large in size that they can easily be seen with the lens power of a dissecting microscope.
Nuclei of these cells are much larger than those of ordinary cells being generally about 25µ in diameter, and chromosomes in nuclei are so large that they are 50 to 200 times as large as chromosomes in other body cells of the organism.
They were first observed in 1881 by E.G. Balbiani in Chironomus and were studied by Korschelt (1884) and Corney (1884). Heitz and Bauer in 1933 studied these giant chromosomes in Bibio hortulanus larvae, while Painter (1933) described them in salivary glands of Drosophila.
Because of their large size showing numerous strands these are named as polytene chromosomes (name suggested by Kollar) or commonly salivary gland chromosomes. The latter term is a misnomer as these chromosomes may occur in other somatic cells of body besides salivary gland cells.
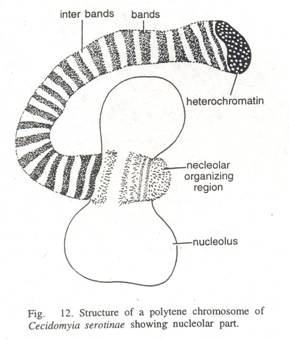
Ultrastructure of giant polytene (poly=many, tene=strands) chromosomes:
It was first investigated by Beermann and Bahr (1954), who observed numerous fine fibrils in the Balbiani rings of Chironomus and estimated that each chromosome contains 1000 to 2000 separate strands (corresponding to the degree of ploidy).
Later Gay (1956) observed strands 200 to 500 A in diameter in sectioned Drosophila salivary chromosomes. The individual fibres in band and interband regions are similar in appearance, but the fibres in the bands exhibit a considerable degree of metaphase-like folding and are much more tightly packed.
Polytene chromosomes get their name from the fact that they are formed by many parallel chromatids, often more than a thousand strands, which do not separate from one another following duplication. Along each chromatid strand some regions of chromatin are tightly coiled and other regions are less coiled, with the result that polytene chromosomes appear to consist of light and dark bands when observed under a microscope.
During larval development, specific areas on polytene chromosomes become uncoiled, forming localized regions called ‘pufs’. Puffs represent regions of active RNA synthesis (transcription). In the puff individual fibres remain continuous across the puff and they become extended as short lateral loops (Bahr, 1954). DNA is concentrated almost entirely in the bands. Protein and RNA is also found in puffs.
Puffing is due to the uncoiling of chromosome fibres which are usually closely folded or coiled in the dense band regions. These fibres then project in the form of loops.
Puffs and Balbiani rings:
During their initial stages of development these bands or interbands of the chromosomes exhibit swellings or puffs. Their appearance depends on the stage of larval development. It is probable that the metabolic activities, required for the formation of puffs, are related to the secretory function of the salivary glands. The formation of this is controlled by certain specific genes and the puffs are related with the active synthesis of RNA and proteins.
This chromosomal RNA differs from the nucleolar and cytoplasmic RNA. The RNA of puffs is also not similar; it differs from each other in chemical composition. Some regions show larger puffs than others. These larger puffing regions are called Balbiani rings.
These rings are formed by the lateral stretching of loops caused by chromonemata. These loops of chromonemata make up Balbiani rings and give the chromosome a fuzzy outlook. The Balbiani rings are rich in DNA and mRNA, and the formation and function of the Balbiani rings are similar to the puffs.
Functions of giant polytene chromosomes:
(1) Main function of the polytene chromosome is to carry genes which ultimately control physiology of an organism. These genes are formed of DNA molecules.
(2) Shifting of heterochromatin in respect to euchromatin produces giant changes called position effects. These effects cause mutations in animals as well.
(3) Heterochromatic regions contain fewer genes than euchromatic parts. Production of nucleolar material is entirely done by heterochromatin.
(4) Chromosomes also help in protein synthesis indirectly. Nucleolus contains RNA, and this RNA serves as a means of transmission of genetic information to the cytoplasm, leading to the formation of specific protein.
[III] Supernumerary chromosomes:
In nuclei of some plants and animals, in addition to normal chromosomes are present one or more accessory or supernumerary chromosomes. Wilson first discovered these in 1905 in hemipteran insect Metapodius.
Since then they have been found in a variety of insects and in a great many higher plants. In Metapodius these are derived from Y—chromosome. More commonly their ancestry is entirely unknown. Generally they are of smaller size and appear to be genetically inert.
Their presence produces little detectable phenotypic expression in the organism. This suggests that structurally they are largely heterochromatic, whereas in Tradescantia species they are euchromatic; the (J-chromosomes in maize are partly heterochromatic and partly euchromatic (Randolph, 1928). When they are present in large numbers in the same nucleus, they reduce the vigour and fertility (Randolph, 1941). They therefore, cannot be considered genetically inert as had been previously supposed.
Supernumerary chromosomes, as a group, are relatively unstable members of a chromosome complement.