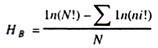In this article we will discuss about:- 1. Dosage Compensation and Sex-Chromatin Bodies 2. Details about Dosage Compensation or Lyon’s Hypothesis 3. Objectives behind the Proposition of Lyon’s Hypothesis and 4. Evidences in Support of Lyon’s Hypothesis.
Dosage Compensation and Sex-Chromatin Bodies:
In man it has been found that Y-chromosomes are genetically inert in comparison to the X-chromosomes and other chromosomes and only a few genes are present in the human Y-chromosome.
From the above discussion on the chromosome numbers of male and female human, it appears that females contain a higher dose of functional gene containing chromosome than males (Female chromosome numbers = 44 + XX and Male chromosome number = 44 + XY).
For many years, geneticists have observed that in some case, female homozygous for the genes in the X-chromosomes do not express a trait more markedly than do hemizygous males. So, it must be a mechanism of “dosage compensation”, through which the effective dosage of genes of the two sexes is made equal or nearly so.
This mechanism of compensating the differential doses of functional sex chromosomes in male and female human is effected by the inactivation of one X-chromosome in the normal female. The genetically inactive X- chromosome or condensed X-chromosome is called hetero-pychnotic X-chromosome or heterochromatin or sex-chromatin body or Barr body (according to the name of the geneticist M. L. Barr who first observed it) or Drum-stick (according to the shape of the inactive X-chromosome).
Of the two X-chromosomes in females, which X-chromosome becomes inactive is a matter of chance, but it should be remembered that once an X- chromosome has become inactivated, all cells arising from that cell will keep the same inactive X-chromosome.
In humans, inactive form of X-chromosome as a Barr-body have been observed by the sixteenth day of gestation. X-chromosome inactivation occurs in human when two or more X-chromosomes are present.
Details about Dosage Compensation or Lyon’s Hypothesis:
The inactive X hypothesis or the Lyon’s hypothesis or the Dosage Compensation is widely known from 1961 which states that only one of the two X chromosomes in the homogametic sex is functional while the other condenses and is inactivated. The X inactivated in some cells would be that from the father, in other cells it would be that from the mother.
Hence any tissue in the body of a woman would be a mosaic of cells which would show dominance of all genes having diffusable products but would remain a fine-grained mosaic for other intracellular differences.
Such a mosaic of cells might be difficult to demonstrate, particularly among rigid tissues, although cells which can be separated and cloned might show antigenic differences. This hypothesis has stimulated many new investigations, some of which are currently being completed.
Objectives behind the Proposition of Lyon’s Hypothesis:
Lyon was impressed by three observations relating to X chromosome:
1. In normal mammalian females, one of the two X’s is genetically inactive in the somatic cells (single active X-hypothesis).
2. Inactivation is random i.e., irrespective of paternal and maternal origin (random inactivation).
3. (a) The inactivation occurs during early ontogeny (early ontogenic differentiation) and (b) The particular X which has thus become inactivated, remains inactive in all the succeeding cell generation (fixed differentiation).
Evidences in Support of Lyon’s Hypothesis:
A. For Single Active X Hypothesis:
1. Bengham (1958) and Russell (1961) noticed that an XO mouse is normal and fertile female indicating that the activity of the single X is sufficient for the normal development of this species.
2. McNeil (1956) recorded the case of “calico-cat” or tortoise-shell cat, usually a heterogygote female with black and yellow patches. Here the dominant X linked coat- color gene producing yellow-color becomes inactivated in some cells, whereas in others this mutant gene produces yellow-color, thus causing a mosaic appearance. Exceptional male “calico” is XXY.
3. X-linked ocular albinism in female heterogygotes causes the mosaic pigmentation of retina showing one active and another inactive X.
4. The late replicating nature of sex chromatin by H3-TdR and very little or no RNA synthesized by Barr body in human body indicate the metabolic activity of one X chromosome.
5. The DNA replication pattern in mammalian females, for example:
Taylor (1960) — in Chinese haunter,
German (1962) — in human being
Mukherjee & Sinha (1966) — in cow etc. shows a late-replicating X (or inactivated) chromosome.
6. In individuals having XXXY or XXX polysomic conditions:
i) There are 2 late Xs.
ii) 2 sex chromatins and
iii) an apparent inactivity of G6PD genes in all but one X chromosome.
From above discussions it is clear that:
B. For Random Inactivation:
1. Ohno and Catlanah (1962) examined that prophase skin cells of “variegated” mouse where dominant autosomal (eighth chromosome) coat color gene had been translocated to an X chromosome as used by Russell. In light colored area the sex chromatin size (obviously the translocated X) was larger than that of sex chromatin in darker patches.
2. Ohno (1963) worked on chinchilla mouse in which the dominant autosomal gene for chinchilla had been translocated onto an X chromosome. In the heterozygote female, the same size variation was observed.
3. Very distinct and ingenious first autoradiographic evidence for random inactivation was provided by Mukherjee and Sinha (1964) who had labelled in vitro the mule (hybrid of female horse + male donkey) leucocytes with H3 thymidine at the terminal part of the “S”-period. In about 50% cells, the metacentric horse X was late replicating while submetacentric donkey — Xs were so in the remaining cells.
Similar observations had been made in several hybrids such as “Zepony” (male zebra + female pony). “Gazel”, “Nikosia” etc.
It has conclusively been noticed that any structurally abnormal X, e.g., ring-X, iso-X, deleted-X etc. is consistently late replicating and, as such, is heavily labelled in autoradiographic experiments.
Random inactivation of either the maternal X or the paternal X chromosome seems to be the rule and the continued typical functioning of the single X-chromosome seems to take place early in development. Selection during embryonic growth may favour those cells which retains the normal X on active status. However, the early decision apparently is final for the inactivation of the one of the two X chromosomes.
C. For Early Embryonic Differentiation:
1. Graham (1954) while surveying the sex chromatin, a various species of mammalian females showed the absence of this structure in early embryos.
2. Despite its absence during early ontogeny, Barr bodies are noticed in late embryos of female cat.
3. Sex chromatins are deleted not earlier than five days in rabbit blastocyst and sixteen and nineteen days in human and macaque embryos, respectively.
4. Hills and Yunis (1966, 68) studied the golden hamster embryos from two-celled stage to about five to six days with H3-TdR and found that late replicating X in female embryos is absent up to eight-celled stage and did identify the late X along the time of implantation at about the fourth day of gestation.
D. For Fixed Differentiation:
1. G6PD, which is much common in Negros and Mediterranean people but is virtually absent in North American whites in deficient activity, is quantitated by its ability for reducing methaemoglobin with the consequent destruction of glutathione.
2. The enzymatic activity is now very conveniently assessed by the action on the substrate and also the detection is made by starch-gel electrophoresis.
3. By “cloning” (aggregation of cells presumably originating from a single progenitor cell), the cells from females, heterozygotes for G6PD activity and employing mostly electrophoretic technique, two cell populations- one enzymatically active and other deficient— were detected.