In this article we will discuss about RNA:- 1. Structure of RNA 2. Types of RNA 3. Replication 4. Antisense RNA 5. RNA as an Enzyme (RIBOZYME) 6. RNA Editing 7. RNA World 8. RNA Interference (RNAi) 9. Peptide Nucleic Acid (PNA).
Contents:
- Structure of RNA
- Types of RNA
- Replication of RNA
- Antisense RNA
- RNA as an Enzyme (RIBOZYME)
- RNA Editing
- RNA World
- RNA Interference (RNAi)
- Peptide Nucleic Acid (PNA)
Contents
1. Structure of RNA:
Ribonucleic acid is a single-stranded structure consisting of long, un-branched polynucleotide chain. RNA normally do not form double helical structure like DNA, but it can often form double helix only when parts of the polynucleotide chain have antiparallel complementary segments and can fold back upon themselves.
RNA, like DNA, is formed of several hundreds or thousands of monomeric units called ribonucleotides which are arranged in a linear sequence and connected together by 3′- 5′ phosphodiester bond.Each ribonucleotide of RNA is composed of pentose sugar, phosphoric acid and nitrogenous base (Fig. 11.1).
The pentose sugar of RNA is ribose sugar. It has an —OH (hydroxyl) group at the 2′ position. The presence of adjacent hydroxyl group of the consecutive ribonucleotide renders RNA more liable than DNA—especially to alkali by which it is degraded with great facility.
In RNA, purine bases comprise mainly adenine (A) and guanine (G), while pyrimidine bases comprise cytosine and uracil (U) instead of thymine. Most RNAs, in addition to the four usual constituents, possess a number of minor bases.
There are all possible methyl and dimethyl derivatives of the four common bases and many kinds of adenine derivatives with different acids, including amino acids combined with the 6-NH2 group; then there are pseudouridine, dihydrouridine and di-hydro-cytosine, thiouridines and several others (Fig. 11.2).
In some kinds of RNA 2′-methyl-ribose is common. The presence of the minor bases has greatly facilitated the determination of nucleotide sequences, especially in transfer RNA.
The presence of minor bases is not, however, a prerequisite for sequence determinations. Phosphoric acid is also one of the constituents of the RNA. RNA are not nearly as large as DNAs and exist in the form of a large variety of well-defined molecules. RNA differs from DNA in many respects which are shown in Table 11.1.
2. Types of RNA:
RNA are of three different types performing different functions during protein synthesis. These are: mRNA (messenger RNA), tRNA (transfer RNA) and rRNA (ribosomal RNA). Although all three types occur as single polyribonucleotide strands, they differ in their characteristic molecular weight, sedimentation coefficient (S), number of nucleotide residue and percent of total cell RNA (Table 11.2).
Each of the three major kinds of RNA occurs in multiple molecular forms. Ribosomal RNA exists in three or more major forms. Transfer RNA occurs as many as 60 types. Messenger RNA occurs as many as the number of functional genes present within the cell or organism.
These three types of RNA are non- genetic RNA. It means that RNAs are not genetic material. They are simply products of genes. Besides three major types of RNA, there are some other types of RNAs which will be discussed later.
(i) Messenger RNA:
It is a single-stranded RNA molecule which carries the message or the information from the gene in the chromosomes to the ribosomes, the cytoplasmic sites of protein synthesis. Since mRNA is the direct product of gene, the base sequence of mRNA is complementary to the one strand of DNA. The mRNA is synthesised on DNA by an enzyme called DNA dependent RNA polymerase.
mRNA is heterogeneous in size and stability. The immediate products of gene transcription in eukaryotic nuclei are very heterogeneous and large in size than mRNA, so these immediate products are called Heterogeneous nuclear RNA (hn RNA). This shows that all eukaryotic mRNAs undergo processing between the time of synthesis and the time of translation. This processing occurs within the nucleus.
Prokaryotic mRNAs have a short half-life (2-3 minutes), while Eukaryotic mRNAs have long half-life (4-24 hours). Polycistrcnic mRNA is common in Prokaryotes but rare in Eukaryotes. Prokaryotic mRNA does not undergo any processing like Eukaryotic mRNAs.
However, the decay or degradation of bacterial mRNA occurs which is a very important steps in regulation of prokaryotic gene expression. Much work has been done on the degradation of polycistronic puf mRNA of the facultatively photosynthetic bacteria Rhodobacter capsular which has been taken as a model system to study Prokaryotic mRNA processing and degradation.
This mRNA encodes the pigment binding proteins of the light harvesting antenna complex and other photosynthetic complexes.
This mRNA is found in abundance in the cells and segments of puf mRNA and show different stabilities. Some secondary structures like hairpin loop at 5 end gives high stability of puf mRNA and some acts against 3′-5′ exonucleolytic degradation.
Thus, prokaryotic mRNA undergoes little modification and processing before the process of translation. The mRNA of eukaryotes are derived from the primary gene transcripts by three steps of processing (Fig. 11.3).
These include:
1. The addition of methyl guanosine groups as mRNA caps to the 5′ ends of the molecules.
2. The addition of approximately 200 nucleotide long sequences of adenylate nucleotides as poly-A tail to the 3′ ends of the molecule.
3. Cleavages of large mRNA precursors (pre mRNA) to smaller mRNA molecules.
The first two steps are called RNA processing and the last step is called RNA splicing. RNA processing is a process to generate a mature mRNA, or a functional tRNA or rRNA from the primary transcript. This RNA processing in case of mRNA can be divided into (i) 5′ capping and (ii) 3′ Polyatenylation the whole step of processing takes place in the nucleus.
It occurs shortly after the beginning of transcription. During transcription this primary transcript (hn RNA) becomes associated with proteins which can be seen as larger particles under the electron microscope. The association of proteins with nascent mRNA forms the structure called Ribonucleic protein fibrils.
These particles are not distributed randomly along nascent mRNA but are bound at specific sites where RNA processing is taking place. After that capping of 5′ end starts (Fig. 11.4).
(a) Mechanism of 5′ Capping:
During synthesis of RNA, 5 ends possess a triphosphate. The terminal phosphate is removed by an enzyme Phosphotydrolase leaving the two phosphate groups. Then a GMP is added to this end in a reverse orientation by Guanylyl transferase forming a 5′ 5′ linkage.
Lastly, the terminal inverted Guanosine is methylated at 7 position and the ribose of the nucleotide of the nascent mRNA is also methylated at 2 position by different methyl- transferees. The 5′ end of pre mRNA now has a methylguanosine cap (Fig. 11.5).
The formation of m7G cap at 5 end of mRNA has several functions such as:
1. Prevents digestion from Exonuclease at 5 end.
2. Helps in transport of mRNA from the nucleus to the Cytoplasm.
3. Helps in the initiation of translation of mRNA.
(b) Mechanism of 3′ Polyadenylation:
It has already been stated that 3′ end of mRNA contains a stretch of adenylate residues forming a poly (A) tail. The poly-A tail contains approximately 250 Adenosine residues in mammals and about 100 in yeast. The major signal for the 3′ cleavage for the addition of poly-A is the sequence is AAUAAA.
Cleavage occurs at 10-35 nucleotides downstream from the specific sequence. Another signal is located about 50 nucleotides downstream from the cleavage site. This signal is a GU-rich or U-rich region, (Fig. 11-6).
The sequence AAUAAA in the primary transcript is the recognition site. Next important step in the processing of mRNA is the removal of introns from the Primary transcripts (pre mRNA) which is called RNA splicing. Introns are long sequences of DNA that do not contain any coding regions of gene or, in other words, which do not carry any information for translation into the protein molecule.
These regions interrupt the coding regions of gene and so eukaryotic genes are called Split genes. Introns are present in most of the genes in higher eukaryotes.
Hence the primary transcript (pre mRNA) from DNA contains both coding regions (Exons) and non-coding regions (Introns). Thus the intron sequences are spliced out of the pre mRNA and exons are ligated to form mature mRNA containing only Exons. These mRNA are then transferred to cytoplasm for translation.
The protein complex containing endonuclease is associated with the next step of the processing at the 3′ end of mass which is actually the cleavage side and addition of poly-A tail. After the cleavage, poly-A polymerase adds poly-A tail.
The function of poly-A tail is to protect the mRNA from premature degradation by exonuclease. This poly-A tail again helps in the purification of mRNA from the total cellular RNA by using a column containing bound poly dT. mRNA having poly-A tail binds to poly dT in the column while other RNA will pass through it. Then mRNA can be isolated by changing the ionic strength of the solution.
The cleavages or splicing involved in the conversion of pre-mRNA to mRNAs involved the removal of leader sequences, sequences from the 5′ end to the translation initiation codon and noncoding sequences called introns that are located between coding sequences called exon (discussed later).
Most of the non-ribosomal RNAs produced in the nuclei of eukaryotic cells consists of very large molecule (about 1,000-50,000 nucleotide length). This RNA is called heterogenous nuclear RNA or hn RNA. Most of the hn RNAs are actually pre-mRNAs. But all hn RNAs are not pre mRNA.
(c) First Method of Splicing:
Rapid processing of the giant primary gene transcripts or hn RNA or pre mRNA molecules in the nucleus yields the mRNA. Nuclear pre mRNA splicing is carried out by complex RN A/protein structure called spliceosomes. This is the first method or splicing. These spliceosomes contain a set of small RNA molecules called sn RNAs (small nuclear RNAs) and a set of proteins that are still not completely known.
Five sn RNAs called U1 (Fig. 11.7), U2, U4, U5 and U6 are involved in nuclear pre mRNA splicing as component of the spliceosome. These sn RNAs do not exists as free RNA molecules. Instead, they are present in small nuclear RNA-protein complex called small nuclear ribonucleoprotein or sn RNPs. Excision of introns from pre mRNA or hn RNA is in 2 steps on spliceosome (Fig. 11.8).
In the first step, a precise cleavage occurs at the 5′ end of the intron and a 2′-5′ phosphodiester linkage is formed between the 5′ position of the G at the intron cleavage site and a conserved A residue near the 3′ end of the intron. Exon I is held in place by components of the spliceosome. In the second step, the two exons are joined by a normal 3′-5” phosphodiester bond and the lariat-shaped is released.
(d) Second Method of Splicing:
In this method, pre mRNA is spliced from the same gene in two ways giving different version of mRNA and finally different proteins. Hence, this method is also known as Alternative splicing. In case of Immunoglobins, say mouse IgM immunoglobins, processing of mRNA takes place in such a way that it may produce secretory antibody or soluble antibody and other antibody that bind to the membrane.
This is clear from the processing of DNA or gene of IgM which has been shown in Fig. 11.8. It has many Exons and Introns. It can be spliced in two different ways. When the spliced RNA include the exons S, V, CI, C2, C3, C4 and C it produces secretory antibody after translation. When the splicing pattern includes S, V, CI, C2, C3, C4 and CM, it produces second antibody that binds to the plasma membrane of the cell (Fig. 11.9).
(e) Third Method of Self-Splicing:
In this method interns are removed by the enzymatic activity of RNA i.e. by Ribozyme and not by protein. Two types of self-splicing introns are present such as Group I and Group II. This type was first shown by Tom Cech.
Group I introns:
The important feature of all Group I introns is the presence of several short sequence elements. Of these, four are conserved sequences and are known either as a, B 9L and 2 or as P, Q, R and S. There are eight sequences of complementary elements. Of these, two are known as 9R and 9R’ or as E and E. Another element is known as the internal guide sequence (IGS) and is complementary to 3 border of the 5′ exon.
The sequence complementarity between pairs of elements is responsible for the formation of a complex, of higher order structure having six helical segments. The interaction between 5 exon and IGS is important for 5′ splicing. The model of a Group I intron is shown in Fig. 11.10.
Group I intron was first noticed in 26S pre- rRNA of Tetraphymena (a ciliated protozoa) and it has also been found in pre-rRNA of Physarumpolycephalum, in mitochondrial pre- rRNAs and in pre mRNA of several fungi.
Group II: introns: These are found in mitochondrial and chloroplast genes. This is characterised by the presence of consensus 5′ and 3 splice sequences. There are six hairpin structures in this intron. The conserved sequences are mostly present in the fifth hairpin structure (Fig. 11.11).
(f) Splicing Mechanism of Group I introns:
A common splicing mechanism is found in Group I introns. It requires Magnesium and a Guanosine cofactor and the energy required for the splicing is obtained by trans-esterification process.
The trans-esterification reaction first takes place at 5 end resulting in the breakage of 5′ splice site which is then esterified with 3-OH group of Guanosine co-factor of Exon 1. Now the second trans-esterification reaction breaks the 3 splice site and the 5 terminus of second exon is esterified with 3-OH group of first exon. In this way, the exons are joined and the introns are released in a linear form (Fig. 11.12).
(g) Splicing Mechanism of Group II introns:
This Group II intron is also spliced auto-catalytically, but in a different way from the splicing of Group I introns. This requires Magnesium and Spermidine but no nucleoside factor is needed. There is no net change in the energy, the number of phosphodiester bonds does not change. Only the position of the bond changes.
The splicing reaction takes place by breaking the intron into monomers. The energy is released from the hydrolysis of RNA to make the splicing reactions. The splicing of some Group II intron is controlled by some unclear encoded factors and by maturates in some cases. In this process, introns are released in the form of a lariat.
(ii) Transfer RNA (tRNA):
Transfer RNA are those RNA that transports amino acids to the ribosome where the amino acids are assembled during the translation of genetic message, i.e., protein synthesis. In the literature tRNA have been variously named tRNA for transfer RNA, S-RNA for soluble RNA or supernatant RNA or adaptor RNA.
It constitutes about 10-15% of the total RNA present within the cell. Each tRNA contains approximately 80 nucleotides having a molecular weight of about 25,000.
Transfer RNA has few salient properties:
1. tRNA is a single-stranded polynucleotide chain.
2. It has a characteristic secondary structure.
3. During protein synthesis tRNA recognises the specific codon present on mRNA.
4. It is able to represent only one amino acid to which it is covalently linked.
5. It contains a trinucleotide sequence called the anticodon (Fig. 11.13) which is complementary to the codon representing its amino acid. The anticodon helps the tRNA to recognise the Condon via complementary base pairing.
6. All tRNAs are able to bind in the ‘P’ and ‘A’ sites of the ribosome, where, at one end, they are associated with mRNA via codon-anticodon pairing while, at the other end, the nascent polypeptide is being transferred.
7. Sometimes each amino acid is represented by more than one tRNA. Multiple tRNAs representing the same amino acid are called iso-accepting tRNAs.
8. A factor that contributes to the diversity of tRNAs is the modificator of certain bases to unusual form following synthesis of the molecule.
Structure of tRNA:
All tRNAs form the secondary structure of a clover leaf with four (sometimes five) arms. The secondary structure folds into a compact L-shaped tertiary structure with the anticodon at one extremity and the amino acid at the other. Holley (1968) first of all proposed a clover leaf model and by X-ray de-fraction analysis. S. H. Kim etal (1972,1973) have proposed a three dimensional L-shaped configuration for a phenylalanine tRNA molecule.
According to clover leaf model, the clover leaf structure of tRNA (Fig. 11.14) is maintained by base pairing between short complementary regions. Complementary regions are held together by hydrogen bonds between complementary bases.
The four major arms of tRNA are named for their structure or function. The acceptor arms consists of a base paired stem that ends in an unpaired terminal sequence of CCA which represents the 3′ end.
The other arms consist of H-bounded base paired stem. tRNA molecule occurs in both active and inactive forms. Inactive molecules lack part Or all of the CCA terminal sequence. The terminal A or adenosine residue containing OH groups welcomes the amino acids for attachment with tRNA.
The amino acids are attached to the tRNA by high energy (very reactive) bonds between the carboxyl groups of the amino acids and the 3′ hydroxyl termini of the tRNA. These reactive aminoacyl tRNAs are formed by a specific activating enzyme or amino acyl-tRNA synthetase (Fig. 11.15).
The TΨC arm is named for the presence of Thymine-pseudouridine (Ψ)-Cytosine triplet sequence. Pseudouridine is one of the unusual bases in tRNA.
The anticodon arm consists of hydrogen bonding base-paired stem and a terminal unpaired loop. This loop contains the anticodon sequence.
The D arm also consists of hydrogen bonding base-paired stem and a terminal unpaired loop. It is so named for its content of another modified base dihydrouridine.
The most variable feature of tRNA is the so-called extra arm of V arm which lies between the TΨC and anticodon arms.
Depending on the nature of the extra arm, tRNAs can be divided into two classes:
Class 1 tRNA:
It has a small extra arm consisting of only 3-5 bases. They represent about 75% of all tRNAs.
Class 2 tRNA:
It has a large extra arm. It may be the longest in the tRNA with 13-21 bases.
The function of the extra arm is unknown. By X-ray de-fraction analysis S.H. Kim et al have proposed L-shaped configuration for a phenylalanine tRNA of yeast cells. According to this model the tRNA is a somewhat flattened L-shaped molecule which remains in tertiary structure.
The base-paired double helical stems of the secondary structure are maintained in the tertiary structure but their arrangement in three dimensions makes two double helices at right angles to each other (Fig. 11.16).
The acceptor-stem and the T Ψ C stem form one continuous double helix with a single gap; the D stem and anticodon stem forms another continuous double helix, also with a gap. The region between the double helices, wherein turn in the L-shape is made, contains the T Ψ C loop and the D loop.
The tertiary structure is produced by hydrogen bonding. The hydrogen bond of the tertiary structure are called tertiary H bonds.
tRNA molecules are synthesised at particular locus of the genetic material.
The function of tRNA is to carry amino acids to mRNA during protein synthesis. Each amino acid is carried by a specific tRNA. Since there are about 20 naturally occurring amino acids in every cell for making proteins by different permutation and combination, there must be at least 20 types of tRNAs. E. Coli contains about 30 to 40 different tRNAs.
Higher organisms are found to contain 60 different tRNA molecules. It has, however, been shown that in many cases several tRNA carry the same amino acids due to their affinity for them. The codon of mRNA and anticodon of tRNA are complementary to each other.
On that basis each codon welcomes one tRNA having the complementary base sequence in their anti-codon part. Hence codon-anticodon recognition ratio is 1 : 1. Often one tRNA can recognize more than one codon. This phenomenon can be explained by Wobble hypothesis.
This hypothesis states that the two bases of the mRNA codon and anticodon pair properly, but the third base in the anticodon has some play (or wobble) that permits it to pair with more than one base. Table 11.3 shows that codon- anticodon base pairing at the third position according to Wobble hypothesis.
The Wobble hypothesis predicted the occurrence of three tRNAs for the six serine codons.
Three serine tRNAs have been characterised:
i. The anticodon AGG of tRNA ser1 binds to codons UCU and UCC.
ii. The anticodon AGU of tRNA ser2 binds to codons UCA and UCG.
iii. The anticodon UCG of tRNA ser3 binds to codon AGU and AGC.
Again, several tRNAs contain the base inosine at the third position (Wobble position). This unusual base can pair with adenine, uracil or cytosine in the codon. Codon-anticodon pairing is influenced by modification of the anticodon itself and also by the context of adjacent bases, especially on the 3′ side of the anticodon.
Taking advantage of codon-anticodon Wobble allows vertebrate mitochondria to recognize all codons, compared with the usual minimum 31 tRNAs; this is assisted by changes in the genetic code in mitochondria. Mutation may allow a tRNA to read different codons.
Mutation generally occurs in the anticodon itself. Alternation of its specificity may allow a tRNA to suppress a mutation in a gene coding for protein. Some tRNAs appear to read ‘codons’ of four bases and generate a frame shift. Such effects suggest that codon- anticodon recognition is involved in setting the distance that the ribosome moves in a translocation event.
Pre-tRNA has also undergone processing to become a functional tRNA.
Some of these modifications are:
i. Removal of extra segment at the 5′ end by RNA ase P.
ii. Removal of an intron in the anticodon loop.
iii. Replacement of two U residues at the 3′ end by CCA.
iv. Modification of some bases to Inosine, Dihydrouridine and Pseudouridine.
(iii) Ribosomal RNA (rRNA):
Ribosomal RNA or rRNA is a type of RNA which acts as a structural component of ribosome. It builds up a ribosome in association with ribosomal protein or r-proteins. Ribosomal RNA(rRNA) generally represents more than 80% of the RNA present in cells.
Prokaryotic ribosomes consist of three RNA molecules—16S rRNA in the smaller sub-unit and 5S and 23S in large sub-unit. Eukaryotic ribosome contains four rRNA—18S rRNA in smaller sub-unit and 28S, 5S and 5.8S rRNA in the large sub-units (Fig. 11.17).
Ribosomal RNA is single-stranded, un-branched, polynucleotide chain having 3′ and 5′ ends. But it has a high degree of secondary structure. About 70% of it is double-stranded and helical due to base pairing. These double-stranded regions are formed by “hairpin loops” between complementary regions of the same linear RNA molecules as shown in Fig. 11.18. Each loop contains both duplex stem and unpaired bulges.
The various proteins bind at specific points on these loops and stems. Ribosomal RNA provides a three dimensional matrix to which the various enzymes of protein synthesis machinery bind in an orderly fashion. Ribosomal RNA also participates in protein synthesis by means of its base pairing properties.
The 3′ terminal region of the rRNA seems to be of particular importance. This end of 16S rRNA isolated from E. coli has a Shine and Dalgarno sequence which is complementary to the ribosomal binding site of most prokaryotic mRNA.
The interaction of 16S rRNA and mRNA helps the 30S sub-unit recognise the starting end of the mRNA when binding to it. The 5S RNA has a sequence which is complementary to the TΨC sequence present in all tRNAs. TΨC sequence is essential for the binding of tRNA to ribosomes.
Large number of ribosomes consisting of several rRNA molecules along with some proteins are present in Eukaryotic cells. In mammalian ribosomes, large sub-unit of 60s consists of 49 ribosomal proteins and several types of ribosomal RNAs like 5S rRNA, 5.8S rRNA and 28S rRNA.
Small sub-unit of 40 S consists of 33 ribosomal proteins and 18 S rRNA. Of the total cellular RNA, 80% consists of ribosomal RNA. This large quantity of ribosomal RNA comes through the repetitive sequences of genes encoding rRNA (r DNA) and remains clustered in different regions of the genome.
At the interphase stage, clusters are grouped together to form one or more irregularly shaped structures called Nucleoli. The regions of chromosomes containing rDNA are called Nucleolar organizer. Under the electron microscope, the nucleolus consists mainly of granular and fibrillar structures.
The granular structure is due to the presence of ribosomal sub-units and the fibrillar structure is due to the presence of rDNA templates and rRNA transcripts.
The synthesis of rRNA precursor has been studied in details in amphibian oocytes as these are very large cells (2.5 um in diameter). During the growth of oocytes, large number of nucleoli are seen, indicating the increase or the amplification of rDNA.
For this reason, oocytes are the ideal material for the investigations of rRNA synthesis and processing. When the fibrillar regions of oocyte nucleoli are dispersed and examined under electron microscope, a chain of Christmas tree like structure is seen.
From the DNA strand about 100 or more fibrils are arranged like a branch of a Christmas tree. These fibrils are actually the rRNA transcripts in the process of elongation along with some associated proteins. The length of the fibrils increases gradually from one and of a trunk to other.
The length of DNA between the longest and shortest fibril is called the transcription unit. RNA fibrils also contain some particles, i.e. associated proteins that help to convert the rRNA transcript to final rRNA required for ribosomal sub-unit. It has already been stated that the large sub-unit of ribosomes in
eukaryotic cells consists of 28 S, 5.8. S and 5 S rRNA molecules and the small sub-unit contains only 18 S rRNA molecule, or these rRNAs, 5 S rRNA is synthesised from the separate rRNA precursor outside the nucleolus and other three rRNAs synthesised in the nucleolus are separated from a single transcript by various nucleases.
This cleavage is helped by U3 Sn RNA and other U-rich Sn RNAs. The region of DNA between adjacent transcriptional unit is not transcribable and so it is called Non Transcribed spacer.
There are some peculiarities of pre-rRNA such as:
(a) Large number of nucleotides are methylated, and
(b) Some contain pseudouridine residues.
All these modifications take place after transcription. The processing of rRNA transcript is done with the help of small nucleolar RNAs (sno RNAs) which are associated with specific proteins forming a complex called small nucleolar ribonucleprotiens (sno RNPs). There are other types of proteins present in the nucleolus which help in the formation of two ribosomal sub-units.
5 S rRNA genes of Eukaryotes are located outside the nucleolus. 5 S rRNA genes are present in tandem array as repeating units along with the non-transcribed spacers. This gene is transcribed by RNA polymerase III.
The 5′ end of 5S rRNA undergoes no processing but some extra-nucleotides are removed at 3′ end during processing. Then these 5 S rRNAs are transported to nucleolus to combine with 28 S and 5.8 S rRNAs forming the large sub-unit of ribosomes.
(iv) Viral RNA or vRNA:
Viruses possess either RNA or DNA, but not both. RNA containing viruses show some unusual biological properties having the genetic information permanently encoded in RNA. In some cases the genomic RNA also function as mRNA. RNA viruses are influenza, HIV (AIDS virus) turnip yellow mosaic, tomato bushy stunt, tobacco necrosis and the particularly well-studied tobacco mosaic virus (TMV).
TMV contains a single chain of RNA of about 330 nm length and runs the entire length of TMV rod. It contains about 7,300 nucleotides. RNA is not present in the centre of the hole but intermeshes with the protein sub-unit. It protrudes from one end of the virus. RNA alone is capable of causing infection.
In some of the single-stranded RNA viruses, the chromosomes also serve as viral mRNA; in others transcribed RNA, complementary to the RNA chromosome, serves as mRNA. Virion RNA that can function as mRNA is termed plus strand RNA, while RNA that is complementary to virion mRNA is termed minus strand RNA.
In some groups of RNA viruses (Reo-viruses), a virion contains several RNA molecules—each encoding one or two viral proteins. Virion that exhibit this highly unusual genetic organisation are said to posses a segmented genome.
(v) Mitochondrial RNAs:
13 mRNAs, 22 tRNAs and 2 rRNAs are transcribed by human mitochondrial DNA. Minimal processing is the rule for mitochondrial transcripts. The mRNAs are made with no or very short leader before the initiator AUG codons. It has no internal sequences and some of them have no terminal codon and end in U or UA partial codons which become UAA only upon addition of a poly-A tail.
3. Replication of RNA:
In eukaryotes and prokaryotes, the cytoplasmic RNAs like mRNA, tRNA and rRNA do not replicate. They are simply bio-synthesised from the DNA using it as template.
Viral RNA has served as a model of RNA replication. Replication of viral RNA takes place in the cytoplasm of a susceptible host cell.
Most of the RNA viruses like tobacco mosaic virus (TMV), influenza and poliomyelitis carry single-stranded RNA but Reovirus group contains double-stranded helical DNA.
In certain RNA viruses (Retroviruses eg HIV) the RNA may act as a template for the formation of DNA with the help of an enzyme called reverse transcriptage. The DNA is complementary to the viral RNA. Next, reverse transcriptase synthesizes a second strand of DNA but this one is complementary to the first DNA strands synthesised.
The two DNA strands form a circular double-stranded chromosome that migrates to the nucleus where it becomes linearly integrated in the host chromosome (Fig. 11.19). Then the RNA Polymerase of the host transcribes the integrate viral gene, producing viral RNA for incorporation into new virion.
 Fig. 11.19: Replication of the retroviral RNA (RT = Reverse Transcriptase)
Fig. 11.19: Replication of the retroviral RNA (RT = Reverse Transcriptase)
But in others the RNA appears to act as a self-replicating model. During self-replication in case of virus containing plus strand RNA— the RNA is translated immediately after it enters the host cell; one of the proteins thus produced is a replicase (RNA dependent RNA polymerase) which catalyses the formation of a complementary strand of RNA termed minus strand.
The plus and minus strands form a double-stranded intermediate molecule termed the replicative form (Fig. 11.20). Then, using the minus strand as a template, replicase catalyses the synthesis of new copies of plus strand.
When virion contains minus strand or double-stranded RNA it cannot be translated because they lack the host’s ribosome binding sites. All virions that contain such RNAs also contain replicate molecules which enter the host cell along with the RNA.
These enzymes catalyze the replication of viral RNA through a double-stranded replicative intermediate. In these viruses, additional plus strands are synthesised .from the replicative intermediate to serve as mRNA for the synthesis of viral proteins.
4. Antisense RNA:
In DNA duplex, the strand that bears the same sequence as the mRNA (except for possessing T instead of U) is called the coding strand or sense strand. The other strand which directs synthesis of the mRNA via complementary base pairing is called the template strand or antisense strand (Fig. 11.21).
During transcription of DNA into RNA, the antisense DNA strand produces sense or messenger RNA, while sense DNA strand produces antisense RNA. Messenger RNA is the only transcription product of most genes; however, some genes are regulated by the additional transcription of an antisense RNA from the sense DNA strand.
If an antisense RNA is made, then the antisense RNA and the messenger RNA bind with each other, a variety of factors may then prevent translation of protein: the RNA duplex may be rejected by the ribosomes; it may be degraded by enzymes; it may never leave the nucleus or a base may be modified chemically to become different inosine bases—thereby disturbing the genetic code on the messenger RNA.
Today antisense RNA molecules are valuable research tools which turn off or modify the activity of any given gene indirectly.
Antisense RNA seems to be universal among viruses and bacteria. If an antisense RNA is synthesised in vitro and introduced into the cell, the antisense RNA will bind specifically with a targeted gene’s RNA messenger, thereby interrupting the precise molecular choreography that expresses a gene as a protein. In this way viruses and bacteria regulate some genes during their life cycle.
Nowadays antisense technology is contributing to the birth of a new field—reverse genetics. Classical genetics usually includes the random mutations of all genes in an organism and selects the mutation responsible for specific characteristics; reverse genetics starts with a cloned gene of interest and manipulate it to elicit information.
In 1983 Tomizawa demonstrated that anti- sense RNA against any cloned gene inhibits the translation of its messenger RNA. This observation permits the researchers to inactivate specific genes much as mutation could, but with higher selectivity.
Today recombinant-DNA technology is being exploited to create artificial genetic elements called expression vectors that would make antisense RNA when they are inserted into cells.
In this process an isolated messenger RNA molecule can serve as a template for making a complementary strand of antisense DNA (Fig. 11.22). This DNA strand can, in turn, act as a template for making the sense DNA strand and creating a DNA duplex.
A plasmid or double strand ring of bacterial DNA is cut by restriction enzymes near a promoter region. The DNA duplex can be spliced into the plasmid to create an expression vector.
The effectiveness of this expression vector can be tested in cells. When the promoter of expression vector initiates transcription, the expression vector will make copies of the original messenger RNA.
If the inserted DNA duplex is then cut out of the expression vector with restriction enzymes, it can reinsert itself into the ring with the opposite orientation during transcription, this expression vector will then make antisense RNA.
This concept has been tested in reality for a gene controlling the synthesis of an enzyme thymidine kinase (TK). This enzyme converts Thymidine, a molecular precursor for the T-base in DNA, into a form that can be added to growing strands of DNA.
DNA or mRNA was initially taken from Herpes simplex virus (HSV). The cells where the expression of antisense RNA was tested were obtained from a standard mouse cell- culture line that had a mutated TK gene. When the expression vectors containing the HSV-TK genes or DNA were injected into these mouse cells, the cells began to incorporate thymidine normally in the mutated cell.
This is a routine experiment of recombinant DNA technology. But when the antisense expression vector, constructed as mentioned previously, is injected into the same mutated mouse cell culture line that had previously received HSV- TK expression vectors, the ability of the cell to incorporate thymidine is inhibited (Fig. 11.23).
Now it is clear that antisense RNA can affect cloned target genes inserted into the cell.
It was also observed that antisense TK-RNA from chickens could act only on its own system and inhibit the activity of chicken TK genes. But antisense TK-RNA from a chicken failed to inhibit TK genes from a virus, and vice versa— because chicken TK genes and viral TK genes are dissimilar, their RNA products do not hybridize. These results showed that antisense inhibition can be highly specific.
Application of Antisense RNA:
Antisense RNA technology contributes a lot of information to the understanding of gene function, particularly in the areas of development, cell growth and cell division. Another completely new way of exploitation and application of antisense RNA technology is the creation of mimic mutation.
Flerbert Jackie and his colleagues at the university of Munich first developed mimic mutation in Drosophila embryo with antisense RNA technology. By inactivating specific gene with antisense RNA, they were able to produce fruit flies whose characteristics matched those of flies known to have mutations in those genes. Such phenocopying has also been achieved in more complex organisms including mice.
“Shiverer” is the name to a type of mutant mouse that has a defective nervous-system protein called myelin basic protein. Due to this defect, the myelin sheaths surrounding and insulating the nerve fibres are imperfect and the Shiverer mice shake uncontrollably. When antisense expression vectors against myelin basic protein are inserted into normal mouse embryos, the resulting twice tremble like Shiverer mutants.
Besides phenocopying, entirely new forms can also be created by antisense RNA technology. Antisense expression vectors have been injected into Petunias to inhibit an enzyme that produces pigment in the flower. The resulting flowers shows unusual pigment patterns.
In tomatoes, antisense expression vectors can inhibit the enzymatic reaction that breaks down the cell wall of the fruit’s cell during ripening. Hence antisense expression vector treated tomatoes ripen slowly than the untreated ones. Hence it is possible to create tomatoes that can be transported to a distant place without spoiling.
Antisense RNA technology can be employed against plant viruses to create a disease- resistant variety of tobacco. This new field of reverse genetics is rapidly providing inroads into the understanding of gene function. Today antisense RNA molecules are the valuable research tools.
This technology is now becoming important for its therapeutic application. Eventually, certain diseases are being treated with this technology.
5. RNA as an Enzyme (RIBOZYME):
It was long thought that, in a living cell, every cellular reaction is catalysed solely by protein enzyme, while the nucleic acids (DNA and RNA) contain the information needed for metabolism and reproduction. However, in 1981, Thomas R. Cech and his Coworkers experimentally demonstrated that an RNA molecule can have highly specific catalytic activity and cannot act as an enzyme.
They found that in Tetrahymena thermophile (a ciliated protozoan) a 6-4 kb precursor of ribosomal RNA can catalyze the cutting and splicing which leads to the removal of part of its own length. RNA is not a protein but the Tetrahymena RNA comes close to fulfilling the definition of an enzyme.
One remaining difference was that enzymes operate on other molecules, rather than on themselves—as the Tetrahymena RNA does. For this reason, a new term “ribozyme” has been given for this enzyme—like RNA. It is also observed that a slightly different form of the same RNA can catalyze the assembly of RNAs other than itself and is, therefore, an enzyme in the full sense.
The ribosome is composed of several molecules of RNA along with a variety of proteins. It may be that the RNA of the ribosome rather than its protein is the catalyst of protein synthesis, one of the most fundamental biological activities.
RNA catalysis also has evolutionary implication. Since nucleic acids and proteins are interdependent, it has often been argued that they must have evolved together. The finding that RNA can be a catalyst as well as an informational molecule suggests that, when life originated, RNA may have functioned without DNA or proteins.
Structure of Catalytic RNA:
Normally, RNA is a single-stranded linear structure. If two separate RNA strands containing the appropriate complementary nucleotides are mixed together they can join and make a double-stranded structure. In some cases a single-strand RNA can fold back on itself and form short double-strand region in their complementary part while non-bonded regions loop out.
The RNA isolated from Tetrahymena has a folded structure formed by many double-helical stems and loops (Fig. 11.24). The folded RNA contains weak GU base pair in addition to the stronger AU and GC pairs; their approximate affinities are in the ratio of 1 : 100 : 1,000.
This folded secondary and tertiary structures of the RNA from Tetrahymena has a critical role in the molecule catalytic activity.
Catalytic Activity of RNA:
The RNAs produced from eukaryotic split gene contains a number of functional units called exon. Each exon alternates with nonfunctional parts called intron. When the precursor molecule of RNA containing both intron and exon is processed, the phosphodiester bonds at both ends of intron—called the 5′ splice site and the 3′ splice site—are broken (Fig. 11.25).
As a result, introns are removed and adjoining exons are spliced together to yield a functional RNA.
This type of cleavage and modification of RNA does not take place by means of enzyme. Rather, this reaction is catalysed by RNA molecule that acts on itself. Such splicing or auto-catalytic activity has been shown to occur in rRNA of Tetrahymena, several lower eukaryotes and in a large number of rRNA, tRNA, mRNA precursors in mitochondria and chloroplasts of many different species.
The autocatalytic excision of the intron in Tetrahymena rRNA precursor requires no external energy source like ATP and no protein. Instead, in it takes place a series of phosphoester bond transfers with no bonds lost or gained in the process.
The reaction needs a guanine nucleotide (G) with a free 3′-OH group as a cofactor plus a monovalent cation and divalent cation. This cofactor serves as an attacking group that becomes transiently incorporated into the RNA.
G binds to the precursor RNA containing a specific binding pocket for G (Fig. 11.26) and then attacks the 5′ splice site to form a phosphodiester bond with the 5′ end of the intron. This trans-esterification reaction generates a 3′ OH at the end of the upstream exon.
The 3′ splice site is then attacked by the newly-formed 3′ OH group of the upstream exon. The second trans-esterification reaction joins the two exons and leads to the release of 414 nucleotide intron. Two more rounds of self- splicing takes place.
The 3′ OH of the intron attacks a phosphodiester bond near the 5′ end to form a circle and a 15-nucleotide fragment containing the G that was incorporated in the first step. This 399-nucleotide circle opens into a linear molecule which cyclizes to lose a 4-nucleotide fragment. This circle opens into a linear RNA called L-19 IVS (linear minus 19 intervening sequence) (Fig. 11.27).
Analysis of the base sequence suggested that the 5′ splice site is aligned with the catalytic residues by base pairing between a pyrimidine- rich region (CUCUCU) of the upstream exon and a purine-rich guide sequence (GGGAGG) within the intron (Fig. 11.28).
The intron brings together the guanosine cofactor and 5′ splice site so that the 3′ OH of G can attack the phosphorus atom at this splice site. Another part of the intron then holds the downstream exon in position for attack by the newly formed 3′ OH of the upstream exon. A phosphodiester bond is formed between the two exons and the intron is released as a linear bond.
Significance of base pairing for the catalytic activity of Tetrahymena intron was demonstrated in experiments that destroyed the intron’s capacity to form double-strand regions.
Among the sequence, elements that have a particularly significant role in determining the folded structure of the intron’s core region is shown in Fig. 11.28 (folded structure of rRNA from the protozoan Tetrahymena). The core regions are the one designated 9R’, A, B, 9L and 2.
In the fully functional intron, 9L pairs with 2 to form a double strand region Fig. 11.24. When mutations are introduced into 9L or 2 that change the nucleotide sequences and prevent normal pairing, the intron is no longer capable of splicing itself out of the rRNA precursor. Combining the two sets of mutation reestablishes pairing and restores the catalytic activity of the intron.
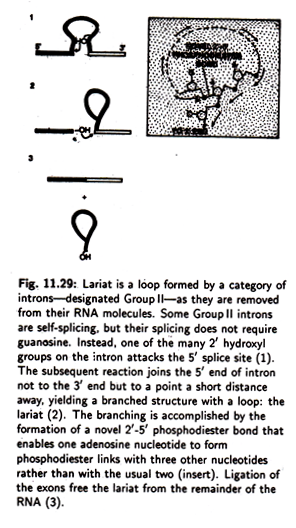
The functional significance of 9R’, A, B, 9L and 2 is further emphasised by the fact that they have been conserved through evolution in all introns related to that of the Tetrahymena pre rRNA which make up a category called group I. Lariat (Fig. 11.29) is a loop formed by a category of introns— designated Group II—as they are removed from their RNA molecules.
Some Group II introns are self-splicing but their splicing does not require guanosine.
Instead, one of the many 2′ hydroxyl groups on the intron attacks the 5′ slice site. The subsequent reaction joins the 5′ end of the intron—not to the 3′ end but— to a point a short distance away, yielding a branched structure with a loop, the lariat.
The branching is accompanied by the formation of a novel 2-5′ phosphodiester bond that enables one adenosine nucleotide to form phosphodiester links with three other nucleotides, rather than with the usual two. Ligation of the exons frees the lariat from the remainder of the RNA.
RNA Acts as a True Catalyst:
Although it was well-established that RNA as an enzymes acts on itself rather than on another molecule, it is now noted that RNA also has many enzyme-like properties. Hence RNA can be considered as an enzyme in its full sense. So the distinction between ribozyme and enzyme is narrowed down.
In 1983 Sidney Altman etal investigated the action of ribonuclease P, a tRNA processing enzyme found both in bacteria and in higher cells. It is an unusual enzyme in that it contains RNA and protein. Both RNA and protein are needed for the nuclease to carry out its work of cutting the tRNA precursor at a specific point under cellular conditions.
Altman et al also proved that the RNA component of ribonuclease P was the catalytic sub- unit. For this proof they used a DNA template to synthesized the RNA sub-unit of the ribonuclease P. The newly synthesized RNA on DNA template in absence of protein molecule, can catalyze the accurate maturation of tRNA precursor in test tube.
Hence this experiment proves without any doubt that the RNA sub- unit acts as an enzyme in true sense. Presumably the protein—which by itself lacks catalytic activity—helps the RNA to function under the condition prevailing in living cell.
Another interesting observation is that a shortened form of Tetrahymena intron has also the capacity to act as a true enzyme even on new substrate. It is noted that the shortened intron is able to cut and re-join the pyrimidine chain just as it cuts and re-joins itself when the right and intact introns are present.
It has now become evident that in some instances, at least, information-carrying capacity and catalytic activity are present in the same molecule: RNA.
6. RNA Editing:
According to Central dogma, the information found in DNA is used to produce final product protein via mRNA. Now it has been found that the information found in DNA is not always faithfully represented in the protein. It has been shown that there is a process in mitochondria and chloroplasts to alter the sequence of the final product by altering at the RNA level. This process is called RNA editing.
It has been found that the cytochrome c oxidase sub-unit II contains Tryptophan residues in animal but in case of plant it is Arginine, although the sequence of cytochrome c oxidase sub-unit gene is the same in both cases. This change of single amino acid causes a radical alteration in the chemical nature of protein as one amino acid is acidic and the other is neutral.
This problem has been solved by sequencing the mRNA from Cytochrome Oxidase gene of plant and animal. It has been found that in the mRNA the cytosine residue has been changed to uridine i.e., CGG is changed to UGG. This is called editing by changing C to U.
The codon CGG is for Arginine and UGG for Tryptophan. This type of editing has been reported in several genes of Chloroplast, Mitochondria and in Protozoa. Although in majority of cases editing involves a C to U transition, reverse modifications also occur. The editing can occur in 0.8% to 5.8% of the nucleotides of a specific transcript and can happen at random.
Other types of editing are found:
Start codons AUG can also be formed from ACG.
Stop codons can be produced from CAG, CAA and CGA codons through editing.
Editing causes change of amino acids from Proline to Leucine, Serine to Leucine and Serine to Phenylalanine.
The slight alternations in the protein structure are sometimes needed for many functional constraints. Thus the significance of RNA editing is done to conserve the genetic code of DNA for any such minor alterations of the protein.
7. RNA World:
The term RNA World was coined first by Walter Gilbert in 1986 on the observations of the catalytic properties of various RNAs. It is also based on the hypothetical stage in the origin of life on earth, assuming that RNA carried out both the storage of genetic information and the catalytic roles in biochemical reactions instead of proteins.
Catalytic RNAs can cleave and ligate phosphodiester bonds. This has been supported by the discovery of the presence of RNA and not protein in the region surrounding the peptidyl transferase centre of a bacterial 50S ribosomal sub-unit.
There is an idea that carbon—containing organic molecules—were produced in the earth over four billion years ago. It was accepted that at its early age the earth was violent with lightning, volcanic eruptions and torrential rains.
There was little free oxygen and no ozone layer to protect the ultraviolet radiation from the sun. The incoming radiation helped to form many reactive molecules. Spectroscopic analysis of radiation has shown that the cosmic spaces were full of interstellar dust containing a variety of combinations of C, H, O, N and S, or Si.
These free radicals interact to form some organic compounds in presence of radiation and electrical discharge. This has been accepted with the demonstration that certain amino acids were present in some meteorites, celestial bodies, which fell in 1969 in Marchison, Australia.
This likelihood of the formation of organic compound in the ancient period was demonstrated by Stanley Miller in his classical experiment. Miller showed, in his experiment, the formation of a variety of amino acids, the building blocks of proteins, and other biological constituents. Besides amino acids and other organic acids, experiments also yielded sugars and bases like purine and pyrimidine.
These were the components of nucleic acids, DNA, RNA and other biologically important compounds. It was speculated that simple organic molecules such as nucleotides could be bind together to form polymers of variables lengths under laboratory conditions. Once a polymer is formed, it can help in subsequent chemical reactions.
Such autocatalytic system is one of the important properties of living matter. In the living cells at present, the autocatalytic properties are found in polypeptides or proteins. But proteins have no self-replicating properties.
RNA has the self-replicating properties because it has been noted that RNA can be used as a template on which a complementary RNA is synthesised such as in influenza virus. RNA acts as a genetic material in many viruses considering the probability of doing important functions like Replication and catalytic properties.
Proteins could not come in the scenario, as they could not replicate, and DNA also has no catalytic function except replication. But RNA has both properties—so the idea came that RNA world did exist at early ages. The idea of RNA world gained its momentum when it was discovered in 1982 that RNA can act as a catalyst with the name Ribozyme.
In Eukaryotic cells, RNA is synthesised as a single-strand molecule and complementary base pairing is found in some regions. This base pairing leads to the folding of molecules forming a three dimensional pattern. It has been found that proteins enzyme can catalyse chemical reactions on the basis of its folding pattern.
Only thing is that RNA consists of four bases only and so catalytic efficiency might be less as compared to proteins. That may be the reason proteins have taken most of the catalytic functions of the present day complex organisms.
Thus RNA has all the properties in performing all functions of primitive cells. Most likely, the size of genes in early cells is short not longer than 10 to 100 nucleotides with the corresponding proteins of short size probably not more than 20 to 30 amino acids.
Modern genes have several thousand nucleotides. As the genetic systems became more complex in present day cells, there were greater advantages in storing information in a separate molecule and also the catalytic function by-more complex molecule than RNA. So, the appearance of DNA has come in the information system of the cell. It is not clear whether DNA came in the RNA World or evolved later.
It may be concluded that RNA preceded DNA in evolution having both genetic, replicative and catalytic properties. This stage in the origin of life i.e. cellular evolution, has been called RNA World. It may be stated that many of the functions performed by RNA in RNA World have been taken later by DNA and protein.
But RNA still remains an intermediary between the two. However, some important reactions are still controlled by RNA giving a belief that RNA World did exist at early time.
8. RNA Interference (RNAi):
A new type of double-stranded RNA has been discovered first in a nematode, Coenorhablities elegans, which has a role in past transcriptional control of gene. As this RNA interferes in the gene expression or, in other words, in gene silencing, this type of RNA is called RNA interference.
This type of RNA has also been reported in other organisms like Trypanosomes, Drosophila and in Amphibians. RNAi has played an important part in producing mutations by degrading the transcripts i.e., mRNA. It can also activate some un-translated mRNA present in the cytoplasm.
The activation of many mRNAs by RNAi will help to produce large number of proteins needed during rapid development of fertilised eggs. It has another function of extending the viability of some mRNA molecules. For example, as the silk gland synthesizes single type of protein, silk Fibroin in large quantities, this RNAi increases the lifetime of fibroin mRNA molecule for several days.
9. Peptide Nucleic Acid (PNA):
This type of nucleic acid can catalyze many chemical reactions like RNA and has the ability to replicate itself. It consists of peptide backbone composed of H (2 amino ethyl) Glycine units to which nucleobases are attached by carbonyl methylase linkers.
It can also serve as a template for the formation of RNA besides its own replication. Bohler and others showed that one type of PNA can produce Guanosine mononucleotides.