The following points highlight the top three methods used for quantitative estimation of microorganisms. The methods are: 1. Total Count of Microbial Population 2. Viable Count of Bacterial Population by Plating Method Principle 3. Viable Count of Bacteria by Drop Technique.
Quantitative Estimation of Microorganisms: Method # 1.
Total Count of Microbial Population:
Total count of microorganisms in any given sample indicates the total number of microbial cells, both living and dead.
The counting can be made by two methods:
(i) Haemocytometer method;
(ii) Breed smear method.
I. Haemocytometer Method of Total Counting of Microbial Population:
Requirements:
1. Haemocytometer,
2. Bacterial suspension,
3. Cover glass,
4. Microscope, etc.
Haemocytometer:
It consists of a:
(i) Thick glass slide having a depression at the centre, on both sides of which there is one groove and
(ii) A cover slip.
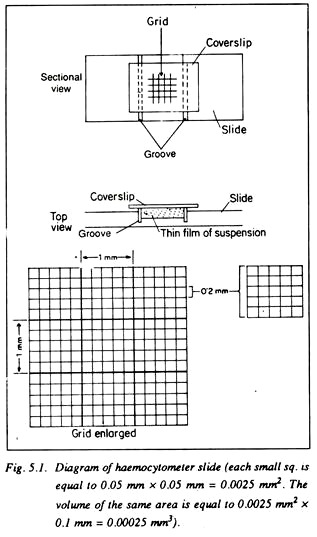
At the centre of the depressed portion there is a grid of definite area which is divided into 9 squares, each with 1 mm. side. The central square is further divided into 25 small squares, each square having a 0.2 mm side.
Each of the squares is divided again into 16 squares each having 0.05 mm. side. In these small squares counting’s are done under microscope. The distance between the cover slip and the central depression is just 0.1 mm. It is thus easy to calculate the volume of suspension where the number of microorganisms is to be counted (Fig. 5.1).
Procedure:
1. The slide and cover glass are cleared by alcohol.
2. A drop of bacterial suspension (10-4 dil) is added by micropipette on the marked area.
3. A cover glass is placed on the suspension and pressed to remove excess suspension and allowed 2 minutes for the cells to settle and then examined under microscope.
4. Then counting the cell numbers per Small Square may be made in 30 – 50 small squares separately.
The area of small square = 0.05 × 0.05 = 0.0025 sq. mm.
Volume of small square = 0.0025 × 0.1 mm. = 0.00025 Cu. mm.
Average no. of cells per small square = x
The dilution of sample =104
So, total cell number = x × 1/0.00025 × 104 cells/ml of sample
II. Total Count by Breed Method:
Requirements:
1. Glass slide,
2. Diamond scratcher,
3. Scale, needle, cover glass etc.
4. Bacterial suspension,
5. Flame, pipettes,
6. Rose bengal or Carbol fuchsin stain,
7. Microscope, etc.
Procedure:
A clear glass slide is marked by diamond scratcher. The marked area is 1 sq. cm. Then the slide is properly cleaned. A drop of original or diluted (10-2, 10-3) bacterial suspension is taken by pipette and 0.1 ml suspension is poured on the marked area.
Then the suspension is slowly spread within the marked area by a needle and dried in air. Finally the dry smear is heat fixed and stained by single stain. The bacterial population is then examined under microscope.
Calculations:
The field area of study under oil immersion objective = πr2 (r = radius of the field of vision).
Average No. of microbes per field area = x. Total number of microbes present in 1 sq. cm area = x × πr2 × 1 cells
0.1 ml of diluted sample (10-2) contains bacteria = x × πr2 × 1 cells
1 ml of original sample thus contains = πr2 x × x 1 x 10 x 102 cells
Quantitative Estimation of Microorganisms: Method # 2.
Viable Count of Bacterial Population by Plating Method Principle:
By plating method the number of viable cells of a bacterial suspension can be determined. This counting may not be exact, since more than one bacterium together may form a colony looking like a single bacterium. That is why in this method we have to consider that each colony has been produced by a single bacterium.
Requirements:
(i) Broth culture of E. coli (fresh),
(ii) Nutrient agar stabs and plates,
(iii) Sterilised distilled water,
(iv) Sterilised tubes, sterilised petridishes and sterilised pipettes,
(v) Burner, pipette stand, etc.
Procedure:
a. Incorporation Method:
The broth culture is diluted to 10-7 concentrations using sterilised pipettes, tubes and distilled water according to the following schedule:
(i) 1 ml of broth + 9 ml sterilised distilled water = dilution 10-1,
(ii) 1 ml of solution i + 9 ml sterilised distilled water = 10-2,
(iii) 1 ml of solution ii + 9 ml distilled water = dilution 10-3,
(iv) 1 ml of solution iii + 9 ml. distilled water = dilution 10-4,
(v) 1 ml of solution iv + 9 ml. distilled water = dilution 10-5,
(vi) 1 ml of solution v + 9 ml distilled water = dilution 10-6,
(vii) 1 ml of solution vi + 9 ml distilled water = dilution 10-7.
Stabs of nutrient agar (containing 15 ml) are melted in a water-bath and then the temperature is brought down to nearly 42-45°C. Then 1 ml of organism suspension of 10-5 to 10-7 dilution is taken by a pipette and poured within the stab aseptically.
For thorough mixing the stab is put on the vortex. After mixing, the stab is poured into a sterilised petridish aseptically near the flame of a burner and is allowed to solidify. After solidification, the plate is kept in an incubator in inverted position (Fig. 5.2.).
Observation:
After 2-4 hours of incubation, the plates are examined and the number of colonies are counted and tabulated:
Results:
b. Spreading Method:
Original broth suspension dilutions made up to 10-7 dilution are used. Nutrient agar medium is melted and poured in sterilised petridishes and the plates are then allowed to solidify. After about 1/2 an hour of media pouring, 0.5 ml of 10-5 to 10-7 dilution suspension is taken and placed in the plate aseptically in separate petridishes.
Then a glass spreader is sterilised by burning and with it the suspension is spread carefully and aseptically over the media within petridish. The plates are kept within an incubator in inverted position for 2 – 3 days for the appearance of colonies. Each treatment is replicated.
Observation:
After 24 – 48 hours of incubation, the plates are observed and the number of colonies is counted. The readings are placed in a tabular form.
Results:
Quantitative Estimation of Microorganisms: Method # 3.
Viable Count of Bacteria by Drop Technique:
This technique for estimation can be used:
(i) To compare the number of colonies appearing in different dilutions;
(ii) When frequent counting is necessary and
(iii) To minimise labour by reducing the use of a number of plates.
Requirements:
1. Petridish,
2. Pasteur pipette,
3. Bacterial suspension,
4. Glass marking pencil,
5. 9 ml sterilised distilled water tubes.
Procedure:
A series of dilutions of the sample arc prepared. Then 3 sterilised petridishes arc taken and molten agar medium (GM – 11) is poured into them and allowed to dry. Each plate is then divided into several segments by marking over the lid with a glass marking pencil.
These segments arc marked for different dilutions. A Pasteur pipette is taken and the volume of each drop is then calibrated. Now the plates are arranged, sample is drawn by the pipette from highest dilution first (say 10-7), the segment of the plate marked 10-7 is brought nearer to you, the lid is lifted on one side and a drop of suspension is delivered from the pipette aseptically on the marked segment.
In this way, suspensions of bacteria of different dilutions arc separated by plates on marked areas of the Petridis.
Then the drops are allowed to dry and the plate is incubated at 30 – 37°C for 2 days. (Fig. 5.3).
Observation and Calculations:
The colonies developed from each dilution are counted after 2 days incubation and viable count of bacterial suspension is then calculated.
The drop volume of a Pasteur pipette is determined by the formula: V = 2rj/dgQ (radius of the pipette – r, surface tension of the liquid – j, density of the liquid – d, gravity – g, correction factor of the neck of the drop – Q). One drop is about = 2/200 = 0.01 ml. Suppose, on incubation, you have found that an average of 50 colonies have developed.
Then the number of organisms present in the original sample may be indicated by the following calculation:
The drop volume of the pipette used = 0.01 ml;
0.01 mm of 10-4 diluted sample contained an arrange of 50 colonies. Therefore, original sample contained
= 50 × 1/0.01 × 104 cells/ml = 5 × 107 cells/ml.