List of nine important antimicrobial agents:- 1. Phenol and Phenol Derivatives 2. Alcohols 3. Halogens 4. Heavy Metal and their Compounds 5. Aldehydes 6. Gaseous Agents 7. Dyes 8. Detergents 9. Quaternary Ammonium Compounds.
Antimicrobial Chemical Agent # 1. Phenol and Phenol Derivatives:
Phenol is obtained by distillation of coal-tar. It is credited to be the first disinfectant used in the form of carbolic acid by Joseph Lister (1867) to create antiseptic conditions during surgery. Today pheno and phenol derivatives (phenolics or phenolic compounds) such as cresols, xylenols and orthrophenylphenol are used as disfectants in laboratories and hospitals.
Phenol is very effective as 5% aqueous solution of this chemical rapidly destroys the vegetative cells of microorganisms; endospores show much more resistance and are no affected. Phenol has the distinction of being the standard against which other disinfectants of a similar chemical structure are compared to determine their antimicrobial activity.
The procedure applied is referred to as phenol- coefficient method.The antimicrobial activities of phenol are greatly increased by various substitutions in the phenol ring structure resulting in variety of phenolic compounds.
These compounds are the -alkyl and -chloro derivatives and the diphenyls. Phenol is rarely used directly during these days because it is very expensive; the phenolic compound, on the other hand, is cheap and more effective hence preferred over phenol.
Phenol and Important Phenol Derivatives:
As stated, the antimicrobial activity of phenol is greatly increased by the addition of chemical substitutions in the phenol ring structure. The chemical structures, properties and applications of phenol and some important phenol derivatives are shown in Table 22.1.
Although the specific mode of action of phenol derivatives is not clearly known, there is consensus that these compounds cause physical damage to plasma membrane of microbial cell. As a result, cell contents leak out and the microorganisms die. Scientists claim that the phenol derivatives may also result in precipitation of proteins, inactivation of enzymes, and leakage of amino acids from the microbial cells.
Antimicrobial Chemical Agent # 2. Alcohols:
The two most popular alcohols used in sterilization are ethanol (ethyl alcohol) and isopropanol (isopropyl alcohol). Methanol (methyl alcohol) is also germicidal but is not generally used as it is highly poisonous and may harm the user.
The fumes of methanol may cause permanent damage to the eyes. Ethanol is effective in concentrations between 50-90%; the ideal concentration is 70% which kills all the vegetative cells but not spores (i.e., the alcohols are not sporicidal).
It has been found that the endospores of Bacillus anthracis have survived for more than 20 years in ethanol. Alcohols are effectively used to reduce the microbial flora of the skin and for the disinfection of clinical oral thermometers. Alcohols can also damage viruses by mechanically removing them by detergent action.
Alcohols may cause dehydration and turn to be microbistatic. It is because the dehydration brought about by alcohol may interfere with the microbicidal action as the surface dehydration of membrane may affect the penetration of alcohols into the cell interior. This alteration does not take place with alcohol of 70% concentration hence the latter considered to be very effective microbicide.
Mode of Action:
Alcohols are protein denaturants. This property of alcohols may, to a large extent, account for their antimicrobial activity. Alcohols are also solvents for lipids, and hence they may damage lipid complexes in the cell membrane.
They are also dehydrating agents. This may account for the relative ineffectiveness of absolute alcohol on “dry” cells; it is possible that very high concentrations remove so much water from the cell that the alcohol is unable to penetrate.
The serve dehydration occurring under these conditions would result in a bacteriostatic condition. Some of the effectiveness of alcohol for surface disinfection can be attributed to its cleansing or detergent action which results in mechanical removal of microorganisms.
Applications:
Alcohol is effective in reducing the microbial flora of skin and for the disinfection of clinical oral thermometers. Alcohol concentrations above 60% are effective against viruses; however, the effectiveness is influenced considerably by the amount of extraneous protein material in the mixture. The extraneous protein reacts with the alcohol and thus protects the virus.
Antimicrobial Chemical Agent # 3. Halogens:
A halogen is any of the five elements (fluorine, chlorine, bromine, iodine and astatine) in group VII A of the periodic table. They exist as diatomic molecules in the free state and form salt like compounds with sodium and most other metals. Chlorine or iodine is the important halogens used as potent antimicrobial agents either in their free form or in the form of their compounds.
Chlorine and its compounds:
Chlorine is most widely used in gaseous form or as its compounds. Chlorine in gaseous form is difficult to handle unless special equipment is available to dispense it. Hence, chlorine gas is used in large scale operations only because it is feasible to install such special equipment for safe, handling in the large scale operation plant.
Many chlorine compounds are now available which can be used more conveniently than free chlorine and which, under proper conditions of use, are equally effective as the free chlorine. Calcium and sodium compounds of chlorine in the form of calcium hypochlorite [Ca(OCl)2; also known as chlorinated lime] and sodium hypochlorite (NaOCl) are popular hypochlorite used in small scale operations.
In addition to hypochlorides, there are another compounds of chlorine grouped as chloramines. Chloramines are more stable than the hypochlorites in terms of prolonged release of chlorine and are often used as disinfectants, sanitizing agents, or antiseptics. They are characterised by the fact that one or more of the hydrogen atoms in an amino group of a compound are replaced with chlorine. Monochloramine (NH2Cl), chloramine – T, and azochloramide are the examples of chloramines.
Mode of action:
Chlorine acts upon microbial cells through the hypochlorous acid formed as a result of the reaction between free chlorine and water. Hypochlorites and chloramines, however, undergo hydrolysis resulting in the formation of hypochlorous acid. The fate of the hypochlorous acid formed during each instance is the same; it undergoes decomposition resulting in HCl and oxygen.
The oxygen released during decomposition of hypochlorous acid (called nascent oxygen) is a strong oxidising agent. It acts on cellular constituents of microorganisms and results in their death. The killing of microbial cells by chlorine and its compounds is also due in part to the direct combination of the cell membranes and enzymes.
Applications:
(i) The compressed gas of chlorine in liquid form is used in sterilizing water in municipal water supplies and also in sewage treatment plants at the rate of 2 ppm,
(ii) Chlorine compounds are used in small scale operations, Calcium hypochlorite (chlorinated lime) is the best and cheapest disinfectant used in dairies, slaughter house, cellars, residential places for floor cleaning,
(iii) Solutions of sodium hypochlorite of 1% concentration are used for personal hygiene and as a household disinfectant; of 5-12% concentration as household bleaches and disinfectants and as sanitizing agents in dairy and food-processing industries, and
(iv) Chlorine compounds are also used to disinfect open wounds and to treat athlete’s foot and other infections.
Iodine and its Compounds:
Iodine in its form appears bluish-black crystalline importing a metallic luster. It is poorly water soluble but readily soluble in alcohol and aqueous solutions of potassium or sodium iodide. Iodine is a highly effective bacterial agent and is unique in that it is effective against all kinds of bacteria.
Iodine is also sporicidal but the rate at which the spores are killed is markedly influenced by the condition under which they are exposed, e.g., amount of organic material and the degree of dehydration. However, the iodine is highly fungicidal and to some extent virusidal.
Iodine is credited to be one of the oldest and most effective antimicrobial agents and is traditionally used as ‘tincture of iodine’, the latter usually being 2% more iodine in a water-ethanol solution of potassium iodide.
More recently, iodine has been complexed with organic compound to form iodophore. Iodophores are water soluble, stable, non-staining. They release iodine slowly into the medium and lower the surface tension of the solution. Some popular iodophore compounds are Wesiodyne, Ioclide, Betadine, etc.
Mode of action:
The mode of action of iodine and its compounds is not clearly understood. It is considered that iodine, being an oxidizing agent, has property to irreversibly oxidize and thus inactivate essential metabolic compounds such as proteins with sulfhydryl groups. It is also claimed that the antimicrobial action of iodine may involve its reaction (halogenation) with tyrosine units of enzymes and other cellular proteins that require tyrosine for activity.
Applications:
(i) Tincture of iodine is applied as an antiseptic in households and hospitals to disinfect wounds, cuts, and scratches, and
(ii) Iodophores are used in hospitals for preoperative skin degerming and in hospitals and laboratories for disinfecting. The iodophores are strongly recommended for wide use because of their being nontoxic, non-staining and odourless.
Antimicrobial Chemical Agent # 4. Heavy Metal and their Compounds:
Most of the heavy metals and heavy metal compounds or metallic salts have some degree of toxicity for microorganisms. For many years the ions of heavy metals were used as germicides but, more recently, these have been super-ceded by other less toxic and more effective heavy metals and their compounds.
The most toxic heavy metals are the mercury, silver, and copper and the least toxic are sodium and potassium. Some common compounds of mercury, silver and copper, and their applications as antimicrobial agents are summarized in Table 22.2.
Mode of Action:
Heavy metals and metallic compounds combine with proteins, often with their sulfhydril(SH) groups and inactivate them. An example of such reaction is shown in Fig. 22.1. High concentration of metallic salts, particularly those of mercury, silver and copper coagulate cellular proteins that results in damage or death of the microbial cell. Metallic salts may also precipate and in high concentrations may cause the death of a microbial cell.
Antimicrobial Chemical Agent # 5. Aldehydes:
Amongst the aldehydes (general formula R – CHO), several of the low molecular weight aldehydes are antimicrobial. Two aldehydes, formaldehyde and glutaraldehyde, are the most effective and are most commonly used to kill spores hence are sporicidal.
These two aldehydes their chemical formula, and their properties and applications are given in Table 22.3:
Mode of Action:
Both of the commonly used aldehydes, formaldehyde and glutaraldehyde, are highly reactive molecules that readily combine with vital organic nitrogen compounds such as nucleic acids and proteins and inactivate them, probably by cross-linking and alkylating molecules. The inactivation of nucleic acids and proteins disrupts the function of cell organelles and, as a result, the cells are killed.
Antimicrobial Chemical Agent # 6. Gaseous Agents:
Sterilization by means of gaseous agents is effective and practical for those kinds of objects that are damaged by heat. Examples are the medical equipment’s (e.g., plastic syringes, blood transfusion apparatuses, catheterization equipment’s) and laboratory wares (e.g. plastic pipettes, Petri dishes).
The main gaseous agents currently used for sterilization of aforesaid objects are ethylene oxide, betapropiolactone, and formaldehyde.
Ethylene Oxide:
Ethylene oxide (C2H4O) is a relatively simple organic compound, and by far, is the most effective and useful gaseous antimicrobial agent known. This compound exists as liquid at temperatures below 10.8°C (51.4°F) and vapourizes rapidly when the temperature moves up. The vapour of ethylene oxide is highly inflammable even in low concentrations.
This is a demerit of ethylene oxide which is overcome by preparing mixtures of ethylene oxide- carbon dioxide or ethylene oxide – Freon. These mixtures are nonflammable without any alteration in antimicrobial activity of ethylene oxide, and are now available commercially.
The CO2 and the Freon merely serve as inert diluents which prevent flammability. Ethylene oxide vapour is highly toxic to viruses, bacteria and fungal cells and also to bacterial endospores. An outstanding and desirable feature of ethylene oxide is its power of penetration as it successfully passes through and sterilizes quite large packages of materials, bundles of cloths, and even plastic wraps.
However, ethylene oxide vapour is explosive and toxic to man hence is used under pressure in a special ethylene oxide sterilizer that very much resembles an autoclave in appearance.
Effective usage of ethylene oxide requires careful control of concentration of the gas, temperature, and moisture, and the modern autoclaves are equipped with controls to maintain the desired concentration of ethylene oxide and the proper temperature and moisture.
Applications:
Since pure ethylene oxide is explosive, it is usually supplied in a 10-20% concentration mixed with either CO2 or dichlorodifluoromethane. As stated earlier, the concentration of ethyloxide, temperature, and moisture influence the rate of sterilization.
The applications of ethylene oxide are:
(i) Ethylene oxide has been established as an effective sterilizing agent for heat- and moisture-sensitive objects. It is used to sterilize the items such as disposable petri dishes and syringes, heart-lung machine components, sutures, catheters, spices, biological preparations, soil, plastics, and contaminated laboratory equipment’s.
However, a clean object from amongst the above can be sterilized if subjected to ethylene oxide for 5-8 hours at 30°C or 3-4 hours at 54°C when the relative humidity is maintained at 40-50% and ethylene oxide concentration at 700 mg/iitre, and
(ii) Ethylene oxide has been used in space programme by both USA and Russia for decontaminating spacecraft components.
Mode of action:
Ethylene oxide is effective against microorganisms by means of its alkylation reactions with enzymes and other proteins. Alkylation reaction causes replacement of an active hydrogen atom from carboxyl amino or sulfhydryl group, with an alkyl group. In this reaction the ring in the ethylene oxide molecule splits and attaches itself at the place where the hydrogen was originally. This reaction inactivates an enzyme possessing a sulfhydryl group.
The alkylation reaction is the following:
Beta-propiolactone (β-propiolactone; BPL):
β- propiolactone (BPL) is a colourless liquid at room temperature.
Its boiling point is high (154.9°C) and its chemical formula is the following:
This compound is a broad spectrum microbicide and kills bacteria, fungi and even viruses. Bacterial endospores also cannot survive when treated with BPL. The letter is occasionally employed in its gaseous form; the gas becomes liquid at 20°C, which is used to sterilize vaccines and sera. BPL is not flammable like ethylene oxide but is a vesicant and lachrymator and consequently must be handled with care.
It destroys microorganisms more readily than ethylene oxide but lacks penetrating power of ethylene oxide. Although BPL is considerably more active against microorganisms, its use is restricted in comparison to ethylene oxide due to its low penetrating power coupled with its alleged carcinogenic properties.
Antimicrobial Chemical Agent # 7. Dyes:
Two groups of dyes, aniline (triphenylmethane) dyes and acridine dyes that possess antimicrobial properties are often used by microbiologists.
These dyes are the following:
Aniline (triphenylmethane) Dyes:
Aniline dyes include brilliant green, malachite green, and crystal violet. Gram -positive bacteria are more susceptible to high dilution of aniline dyes than are gram-negative ones. Malachite green inhibits gram-positive Staphylococcus areus at a concentration of 1:1,000,000 while its concentration of about 1:30,000 is required to inhibit gram-negative Escherichia coli.
Crystal violet inhibits gram-positive cocci at a concentration of 1:2,00,000 to 1: 3,00,000, whereas 10 times more of this concentration is required to inhibit E. coli.
However, the activity of aniline dyes is inhibited by the presence of organic matter like pus hence these dyes show no activity against pus forming bacteria like tubercle bacilli (Mycobacterium tuberculosis, bacilli bacteria that cause tuberculosis). Therefore, the addition of malachite green to Lowerstein-Jensen medium makes it selective for the isolation of tubercle bacilli.
Mode of action:
The mode of action of aniline (triphenylmethane) dyes is uncertain. Some speculate that they interfere with cellular oxidation processes, whereas others claim that these dyes interfere with the synthesis of the peptidoglycan, the main constituent of gram-positive bacterial cell walls.
Applications:
(i) Aniline dues are used extensively as skin and wound antiseptics,
(ii) Use of high dilutions of aniline dyes can make certain culture media selective for specific bacteria. Media of this kind are often used to detect E. coli because such a detection is important in public health microbiology,
(iii) Aniline dyes can also be used for identification of bacteria on the basis of varying degree of susceptibility of bacteria to different concentrations of the dyes. For example, three species of Brucella can be distinguished by their degree of susceptibility to aniline dyes, and
(iv) Crystal violet can be used as fungicide because its 1:10,000 concentration is lethal for Monilia and Torula.
Acridine Dyes:
Acridine dyes include acriflavine, proflavine, euflavine and aminacrine. These dyes exhibit selective inhibition against bacteria, particularly staphylococci and gonococci. Acridine dyes possess little, if any, antifungal activity and, at present, have less application than before the advent of antibiotics and other chemotherapeutic agents.
Mode of action:
Acridine dyes interfere with the synthesis of nucleic acids and proteins in both bacterial and mammalian cells.
Applications:
Acridine dyes are used to some extent for the treatment of burns and wounds and for ophthalmic application and bladder irrigation. Since these dyes have been found to affect the mammalian cells also, their use is very much reduced at present.
Antimicrobial Chemical Agent # 8. Detergents:
Detergents (L.detergere = to wipe off or away) are organic molecules that serve as wetting agents and emulsifiers because they possess both polar hydrophilic and nonpolar hydrophobic ends.
Detergents solubilize otherwise insoluble residues and are very effective cleaning agents. They are different than soaps, which are derived from fats. Since soaps are not very efficient in hard water, new synthetic detergents or surfactants have been developed that prove superior to soaps.
Synthetic detergents do not form precipitates in alkaline or acid water, nor do they produce deposits with minerals occurring in hard water. These detergents are used in laundry and dishwashing powders, sampoos and other washing preparations, and some of them are highly bactericidal.
Chemically, synthetic detergents may be classified into following categories:
(i) Anionic:
Anionic detergents are those the detergent property of which resides in the anion. The examples are a soap [(C9H19COO)_Na+] or sodium lauryl sulfate [(C12H25OSO3)– Na+].
(ii) Cationic:
Cationic detergents are those the detergent property of which resides in the cation. Ceepryn (cetyl-pyridinium chloride) is he example and its chemical formula is the following.
(iii) Nonionic:
These detergents do not ionize, i.e., there is no ionization in the liquid. Nonionic detergents do not possess significant antimicrobial properties.
Although anionic detergents possess some degree of antimicrobial properties, only cationic detergents are effective disinfectants. The most popular of cationic disinfectants are quaternary ammonium compounds.
Which are described separately following this section:
Mode of action:
Detergents mechanically remove the microorganisms from the surfaces (e.g., skin, dirty cloths) on which they are applied. They reduce surface tension and thereby enhance the wetting power of the water in which they are dissolved.
Soapy water emulsifies and disperses oils and dirts and, as a result, the microorganisms become enmeshed in the detergent’s lather and are removed by the rinse water. However, a number of compounds have been incorporated into detergents to increase their microbicidal activity.
Antimicrobial Chemical Agent # 9. Quaternary Ammonium Compounds:
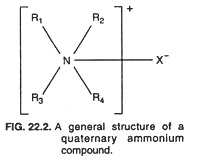
Quaternary ammonium compounds, as mentioned in preceding section, are the most popular cationic detergents. They are characterized by a positively charged nitrogen and a long hydrophobic aliphatic chain. Quaternary ammonium compounds are basically similar in structure to the ammonium chloride but with some modifications.
The characteristic general structure of a quaternary ammonium compound is shown in Fig. 22.2 in which the R1 R2, R3 and R4 groups are carbon groups linked to the nitrogen atom, and the X– is a negatively charged ion such as Cl– or Br–.
Quaternary ammonium compounds are very effective disinfectants and, accordingly, a very large number of these compounds of different constitutions have been synthesized and evaluated for their antimicrobial properties. Some of the important quaternary ammonium salts are: zephirol or zephiran (benzalkonium chloride), ceepryn (cetylpyridium chloride), diaparene chloride, etc. (Fig. 22.3). 
Quaternary ammonium compounds possess an exceptionally high antimicrobial power. They are very effective against gram-positive bacteria and also quite active against gram-negative ones. Microbicidal concentrations of QACs range from dilutions of one part in a few thousand to one part in several hundred thousand.
QACs possess the ability to manifest microbistatic action for beyond their microbicidal action. For convenience, the limit of microbicidal action for a given QAC may be at a dilution of 1:30,000, yet it may show microbiostatic action at dilution as high as 1: 200,000.
Mode of action:
Quaternary ammonium compounds render various damaging effects on microorganisms. They denature proteins, interfere with glycolysis, and disrupt microbial membranes. It has been suggested on the basis of experimental results that the most likely site of the damage to the microbial cell is the plasma membrane because the quaternary ammonium compounds alter the vital permeability characteristics of the plasma membrane.
Applications:
Since the quaternary ammonium compounds possess combined properties of antimicrobial activity and detergent action, and some other properties as high solubility in water, low toxicity, non-corrosiveness, and stability in solution, their applications as disinfectants and sanitizing agents are diversified.
Some important applications of these compounds are:
(i) Quaternary ammonium compounds are used for sterilization of surgical instruments, and for control of microorganisms on floors, walls, and other surfaces in hospitals, nursing homes, and other public places,
(ii) They are used as antiseptics in skin ointments, lotions, etc. and as preservative in ophthalmic solutions,
(iii) They are used to sanitize utensils used to prepare food and beverages in restaurants,
(iv) They are used to sanitize surface and certain equipment in food- processing plants,
(v) Quaternary ammonium compounds are also used in dairy, poultry, and fishing industries to disinfect surfaces of equipment and the environment in general.