The following points highlight the top four types of culture media used in the cultivation of anaerobic bacteria. The culture medium are: 1. Special Anaerobic Culture Media 2. Anaerobic Chamber 3. Anaerobic Bags or Pouches 4. Anaerobic Jars.
Type # 1. Special Anaerobic Culture Media (Prereduced Media):
Of all the methods available for the cultivation of anaerobic bacteria, exclusion of oxygen from the medium is the simplest method. During preparation, the liquid culture medium is boiled by holding in a boiling water both for 10 minutes to drive off most of the dissolved oxygen.
Liquid media soon become aerobic thus a reducing agent (e.g., cysteine 0.1%, ascorbic acid 0.1%, sodium thioglycollate 0.1%), is added to further lower the oxygen content.
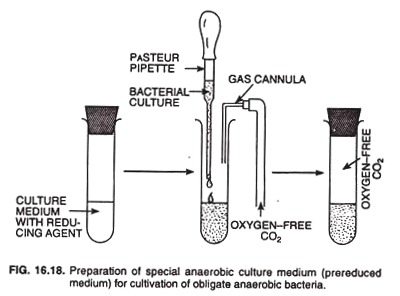
Oxygen-free N2 is bubbled through the medium to maintain anaerobic condition. The medium is then dispensed into tubes, which are stoppered tightly and sterilized by autoclaving. Such tubes can be stored for many months before being used. During inoculation, the tubes are continuously flushed with oxygen free CO2 by means of gas cannula, re-stoppered, and incubated (Fig. 16.18).
Cooked meat broth (CMB; original medium known as ‘Robertson’s bullock-heart medium’) has a special place in anaerobic bacteriology, and thioglycollate broth and its modifications are also very useful. CMB is suitable for growing anaerobic bacteria in air and also for the preservation of their stock cultures.
The inoculum of the bacterium is introduced deep in the medium in contact with the meat. Meat particles are placed in 30 ml bottles to a depth of about 2.5 cm and covered with about 15 ml broth.
However, some other media which can be used for recovering anaerobes are Brucella blood agar, Bacteroides bile aesculin agar, phenylethyl alcohol agar, kanamycin blood agar, etc. Anaerobic bacteria have special nutritional requirements for vitamin K, haemin and yeast extract, and all primary isolation media for anaerobes should contain these three ingredients.
Type # 2. Anaerobic Chamber:
Anaerobic chamber is an ideal anaerobic incubation system, which provides oxygen- free environment for inoculating media and incubating cultures. It refers to a plastic anaerobic glove box that contains an atmosphere of H2, CO2, and N2. Glove ports and rubber gloves are used by the operator to perform manipulations within the chamber. There is an air-lock with inner and outer doors.
Culture media are placed within the air-lock with the inner door. Air of the chamber is removed by a vacuum pump connection and replaced with N2 through outer doors.
The culture media are now transferred from air-lock to the main chamber, which contains an atmosphere of H2, CO2, and N2. A circulator fitted in the main chamber circulates the gas atmosphere through pellets of palladium catalyst causing any residual O2 present in the culture media to be used up by reaction with H2.
When the culture media become completely anaerobic they are inoculated with bacterial culture and placed in an incubator fitted within the chamber. The function of CO2 present in the chamber is that it is required by many anaerobic bacteria for their best growth. A schematic representation of an anaerobic chamber showing its various parts is given in Fig. 16.19.
Type # 3. Anaerobic Bags or Pouches:
Anaerobic bags or pouches make convenient containers when only a few samples are to be incubated anaerobically. They are available commercially. Bags or pouches have an oxygen removal system consisting of a catalyst and calcium carbonate to produce an anaerobic, CO2-rich atmosphere.
One or two inoculated plates are placed into the bag and the oxygen removal system is activated and the bag is sealed and incubated. Plates can be examined for growth without removing the plates from bag, thus without exposing the colonies to oxygen.
But as with anaerobic jar, plates must be removed from the bags in order to work with the colonies at the bench. These bags are also useful in transport of biopsy specimen for anaerobic cultures.
Type # 4. Anaerobic Jars (or GasPak Anaerobic System):
When an oxygen-free or anaerobic atmosphere is required for obtaining surface growth of anaerobic bacteria, anaerobic jars are the best suited. The most reliable and widely used anaerobic jar is the Melntosh-Fildes’ anaerobic jar. It is a cylindrical vessel made of glass or metal with a metal lid, which is held firmly in place by a clamp (Fig. 16.20).
The lid possesses two tubes with taps, one acting as gas inlet and the other as the outlet. On its under surface it carries a gauze sachet carrying palladium pellets, which act as a room temperature catalyst for the conversion of hydrogen and oxygen into water. Palladium pellets act as catalyst, as long as the sachet is kept dry.
Inoculated culture plates are placed inside the jar and the lid clamped tight. The outlet tube is connected to a vacuum pump and the air inside is evacuated. The outlet tap is then closed and the gas inlet tube connected to a hydrogen supply. Hydrogen is drawn in rapidly. As soon as this inrush of hydrogen gas has ceased the inlet tube is also closed.
After about 5 minutes inlet tube is further opened. There occurs again an immediate inrush of hydrogen since the catalyst creates a reduced pressure within the jar due to the conversion of hydrogen and leftover oxygen into water.
If there is no inrush of hydrogen, it means the catalyst is inactive and must be replaced. The jar is left connected to the hydrogen supply for about 5 minutes, then the inlet tube is closed and the jar is placed in the incubator. Catalysis will continue until all the oxygen in the jar has been used up.
The gasPak is now the method of choice for preparing anaerobic jar. The gasPak is commercially available as a disposable envelope containing chemicals, which generate hydrogen and carbon dioxide when water is added. After the inoculated plates are kept in the jar, the gasPak envelope with water added, is placed inside and the lid screwed tight.
Hydrogen and carbon dioxide are liberated and the presence of a cold catalyst in the envelope permits the combination of hydrogen and oxygen to produce an anaerobic environment.
The outstanding feature of the gasPak system is the disposable gas generator envelope, which does away with the need for a vacuum pump and cylinders of compressed gas; the operation of the jar is consequently very quick and simple. As the standard gasPak jar is not evacuated before use a relatively large volume of water is formed during catalysis.
An indicator should be used for verifying the anaerobic condition in the jar and for this purpose methylene blue is generally used. When it is placed in an anaerobic environment, it is reduced from its coloured oxidized form to a colourless reduced leuco-compound.
Removal of the culture plates from the jar for microscopic examination is the major disadvantage of any anaerobic jar system. This, of course, results in the exposure of the colonics to oxygen, which is especially hazardous to the anaerobes during their first 48 hours of growth. For this reason, a suitable oxygen-free holding system always should be used in conjunction with anaerobic jars.
The culture plates should be removed from the jar and placed in the oxygen-free holding system. From there they should be removed one by one for rapid microscopic examination of colonies, and then quickly returned to the holding system. Plates never should remain in room air on the open bench.