The below mentioned article includes a collection of six experiments that demonstrates the effect of pollution on seed germination.
1. Experiment to study the effect of polluted water on seed germination of any crop:
Requirements:
Polluted water, flask, distilled water, petri-dishes (7), blotting paper, seeds, and tap water.
Method:
1. Take about one liter polluted water in a flask and treat it as of 100% concentration.
2. Prepare its various concentrations (1%, 2%, 5%, 10%, 20%, 30%, and 50%) by adding distilled water.
3. Place a blotting paper in each petri-dish in such a way so that it completely covers the base of each petri-dish.
4. Put a few drops of water to make the blotting paper wet.
5. With the help of a coloured pencil, mark 1%, 2%, 5%, 10%, 20%, 30%, 50% and 100% on different petri-dishes and pour 20 ml each of each of the concentrations of polluted water in respective petri- dishes.
6. Pour only distilled water in one petri-dish and mark it as ‘control’.
7. Place 50 seeds in each petri-dish in such a way so that about the half of the part of each seed remains submerged in water.
8. Wait for about 5 days and then note the number of seeds germinated in each petri-dish.
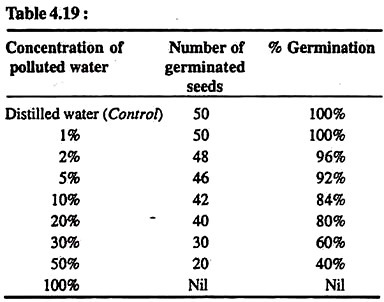
Calculate the germination percentage (Table 4.19):
Results:
100% germination of seeds is observed in distilled water and in very low concentration of polluted water (upto 1%). In the higher concentrations of polluted water, the seed germination decreases with the increase of concentration of polluted water, e.g., it is 96% in 2% polluted water while it is only 40% in 50% polluted water. This decrease in the seed germination is due to the presence of pollutants in the water. This confirms that polluted water shows definite effect on seed germination.
2. Experiment to study the effect of polluted soil on seed germination:
Requirements:
Beakers (6), polluted soil, garden loam soil, seeds, distilled water.
Method:
1. Take six beakers. Fill polluted soil in three beakers and garden loam soil in remaining three beakers. The beakers with garden loam soil will work as control.
2. Sow 10 seeds in each beaker at about 1 cm depth and add a little amount of distilled water in each of them to make the soil moist.
3. Wait for about one week and allow the seeds to germinate. Note the number of germinated seeds in each beaker and calculated the germination percentage.
Observations:
Results:
The data of the above Table 4.20 confirm that polluted soil shows adverse effects on seed germination because there is only 40% germination in polluted soil.
3. Experiment to show the effect of polluted air on seed germination:
Requirements:
Seeds (10), water, petri-dishes (2), blotting paper, fumigation chamber, Na2S, H2SO4with 1 N normality.
Method:
1. Soak the seeds in water and place 10 such seeds in a petri-dish lined with blotting paper.
2. Make the blotting paper moist and place the petri- dish inside the fumigation chamber.
3. Now expose the seeds to SO2 in the fumigation chamber. Allow the SO2 gas to remain in fumigation chamber for quite some time to show its desired effects.
(Fumes ofSO2 can be obtained by reacting known amount of Na2S with IN H2SO4 in a bottle. 5.6 mg of Na2S, when treated with IN H2SO4, will produce 1 ppm ofSO2).
4. Place same number of seeds in another set of petri- dishes lined with blotting paper and moistened with distilled water. Treat them as control.
5. Remove the petri-dish from fumigation chamber after 24 hours, keep it in open and allow the seeds to germinate.
6. Wait for about one week and note the number of germinated seeds in both the petri-dishes.
Observations:
Result:
50% reduction in seed germination in the polluted air confirms that polluted air inhibits seed germination.
4. Experiment to study the effect of saline water on seed germination:
Requirements:
Common salt, distilled water, flask, petri-dishes (9), blotting paper, seeds.
Method:
1. Dissolve maximum possible amount of common salt in distilled water and prepare a concentrated solution. Store this solution in a flask and mark it as 100% saline water.
2. Prepare various concentrations (i.e., 50%, 30%, 25%, 10%, 5%, 2% and 1%) of saline water by adding required quantity of distilled water in 100% saline water.
3. Take eight petri-dishes, cover the base of each of them by blotting paper, add some water on each blotting paper to make them wet and pour 10 ml each of various concentrations of saline water in well-marked petri-dishes (marked as 100%, 50%, 30%, 25%, 10%, 5%, 2% and 1%).
4. In yet another petri-dish, take some distilled water and treat it as control.
5. Place 30 seeds in each petri-dish in such a way that at least half of the part of each seed remains submerged in the solution.
6. Wait for about a week and count the number of seeds germinated in each petri-dish. Calculate the germination percentage.
Observations:
Result:
100% seed germination is observed in distilled water and very low concentration (1 %) of saline water. Seed germination percentage decreases with the increase of concentration of saline water.
5. Experiment to study the effect of water stress on seed germination:
Requirements:
Blotting paper, petri-dishes (4), distilled water, seeds.
Method:
1. Cover the inner basal part of all the petri- dishes by blotting paper.
2. Do not add anything in one set of petri-dish and let it remain dry completely. Place 25 seeds in it.
3. Add small quantity of distilled water in second set of petri-dish so that its blotting paper becomes only just wet. In this petri-dish also place 25 seeds.
4. Place 25 seeds in third set of petri-dish and add sufficient amount of water so that the seeds remain half-submerged in water.
5. In fourth set of petri-dish also place 25 seeds and add large quantity of water so that seeds get completely submerged in water.
6. Wait for about one week and calculate the germination percentage of seeds.
Observations:
Result:
Observations recorded in above Table 4.23 show that seed placed in completely dry petri-dish No.1 show no germination because of non-availability of water.
40% germination of seeds is seen in petri-dish No. 2 because light and air were available to seeds but sufficient water was not available.
100% germination of seeds is seen in petri-dish No. 3 because all basic requirements of seed germination (i.e., light, air and water) are available.
No seed germination is observed in petri-dish No. 4 because water and light are available but no air is available to seeds in completely submerged condition.
It is, therefore, concluded that both over-flooding and completely dry conditions prevent seed germination.
6. Experiment to calculate allelopathic (growth retarding) potential of any weed in relation to seed germination:
Requirements:
Any common weed (e.g., Parthenium hysberopharus), mortar or electric grinder, centrifuge, distilled water, refrigerator, seeds, petri- dishes, blotting paper, beaker, test tubes.
Method:
(a) Preparation of parthenium extract:
Uproot a few plants of any weed (e.g., Parthenium) and remove their shoots from their roots. Crush about 100 gm of shoot part in an electric grinder or mortar and centrifuge this paste for about 15 minutes at 2000 rpm. Take the supernatant and add distilled water to make the final volume to 100 ml. Store this solution in refrigerator and use it as weed extract or Parthenium- extract.
(b) Method of finding out allelopathic (or growth retarding) potential:
1. Take some seeds and soak themin distilled water for about 4-6 hours. The seeds will swell due to imbibition.
2. Take two petri-dishes, place blotting paper to cover the base of each of them and place 25 seeds in each of them.
3. Add Parthenium-extiact in one set of petri-dish to keep the blotting paper completely wet. In another set of petri-dish, add only distilled water and treat it as control.
4. Wait for about one week and then note the number of germinated seeds in each petri-dish. Calculate the germination percentage.
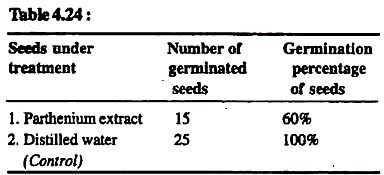
Observations:
Result:
Seeds treated with Parthenium-extract show reduced percentage of seed germination. It is because of the presence of allelopathic or growth retarding alkaloids in the extract of Parthenium plants. When Parthenium plants get decomposed and mixed in the soil, they release these growth-retarding chemicals in the soil. These chemicals inhibit the germination of seeds of crops in such soils.