A mRNA may be associated with several ribosomes (polysomes) in a cell actively engaged in protein synthesis. The process takes place in 4 major steps namely activation of amino acids, chain initiation, elongation and termination. Each step is controlled by specific enzymes and cofactors.
Step # 1. Activation of Amino Acids:
The amino acids are activated by a class of enzymes known as amino acid activating enzymes, or specifically aminoacyl tRNA synthetases of which there is one specific for each amino acid and its tRNA.
The overall reaction is as follows:
The activating enzymes or synthetases range in molecular weight between 100,000 to 240,000; some have a single chain, others are multi-chain enzymes. All of them require Mg++ for their activity. The amino acid is then attached (esterified) to the terminal adenosine residue at the 3′ end of its tRNA in 2 steps.
In the first the amino acid is activated when ATP reacts with the amino acid catalysed by aminoacyl synthetase to form an intermediate aminoacyl adenylic acid-AMP-synthetase complex and pyrophosphate is released. In the second stage the aminoacyl group is transferred from AMP complex to the 3′ end of its tRNA. Following this adenylic acid and aminoacyl-tRNA are released.
ATP + amino acid ⇋ aminoacyl adenylic acid + pyrophosphate aminoacyl adenylic acid + tfRNA aminoacyl-tRNA + adenylic acid
The aminoacyl now becomes esterified by its carboxyl group to the free hydroxyl group at the second position of the terminal adenylic residue at the 3′ end (C-C-A) of tRNA (Fig. 15.23)
The aminoacyl-tRNA synthetases are highly specific for both the amino acid and its tRNA. Consequently, these enzymes must possess at least two binding sites, one for the amino acid, the second for its corresponding tRNA molecule. It is obvious that each aminoacyl-tRNA synthetase is a highly specific enzyme which can select only one amino acid out of 20; and then it selects the correct tRNA for that particular amino acid and no other.
How does an aminoacyl-tRNA synthetase recognize a tRNA? The genetic and biochemical evidence points to a general condition whereby a tRNA and an aminoacyl-tRNA synthetase ‘fit’ each other. In addition, a synthetase might be able to recognise a few recognition points of two or three bases in the tRNA structure.
Events on the Ribosome:
The mRNA after its formation on DNA, migrates out of the nucleus to the cytoplasm where it becomes associated with the 30S subunit of the ribosome. The tRNA molecules loaded with their amino acids (called charged tRNAs) also reach the ribosomes. The 50S subunit of the ribosome has two binding sites for two tRNA molecules called peptidyl (P) and aminoacyl (A) sites.
It is here that the amino acid molecules will be polymerised into a polypeptide chain. In bacteria about 10 amino acids are added to the chain per second, in animals only about 2 per second. The mRNA binds to the 30S subunit at the ribosome-binding site.
Within this site are nucleotide sequences which ensure the correct lining up of mRNA molecules on the ribosomal surface. Every mRNA molecule thus has one ribosomal-binding site for each of its independently synthesized polypeptide chains (Fig. 15.22a).
A ribosome contains only one nascent polypeptide chain bound at its carboxyl end to the tRNA molecule which is attached to its specific site on the ribosome. When the next amino acid is brought by a second tRNA to be linked to the carboxyl end of the chain by peptide bond formation, the first tRNA is released. The ribosome moves over the mRNA and the next triplet of nucleotides comes to lie in position for the next amino acid (Fig. 15.23).
The successive ribosomes of such a polysome carry polypeptide chains at successive stages of completion (Fig. 15.22C). The 5′ end of the mRNA is the first to become attached to the 30S subunit of a ribosome. The number of ribosomes in polysomes is variable.
There may be 5 or 6 as in the case of each polypeptide chain of hemoglobin containing about 150 amino acids. Larger polypeptide chains of about 500 amino acids may have 20 or more ribosomes in a polysome. In E.coli up to 40 ribosomes have been observed in a polysome.
In both prokaryotic and eukaryotic cells, translation begins with the amino acid methionine encoded by the nucleotide triplet AUG. Bacteria may initiate translation using alternative initiation codons such as GUG. When it is present at the beginning of a polypeptide chain, GUG codes for methionine. GUG normally encodes valine.
In bacteria, polypeptide synthesis is initiated with a modified methionine, that is N-formylmethionine, whereas in eukaryotes, unmodified methionine can initiate protein synthesis. Mitochondria and chloroplasts whose ribosomes are similar to those of prokaryotes use formylated methionine to initiate translation.
Prokaryotes and eukaryotes have different signals that identify initiation codons. The initiation codons in bacterial mRNA are preceded by a specific Shine-Dalgarno sequence that aligns the mRNA on the ribosome for translation. Alignment takes place through base pairing with a complementary sequence near the 3′ terminus of 16S rRNA in the small subunit of the ribosome.
The base pairing mechanism allows bacterial ribosomes to initiate transcription not only at the 5′ end of the mRNA, but also at the multiple internal initiation sites of polycistronic messages. In eukaryotes in contrast, ribosomes recognise mRNAs by binding to the 7-methylguanosine cap at the 5′ terminus.
The ribosomes then traverse the m RNA downstream of the 5′ cap until they encounter the AUG initiation codon. Eukaryotic mRNAs do not have the Shine-Dalgarno sequence, and translation is initiated at a site determined by the ribosome traversing the mRNA from the 5′ terminus.
The First Step in Translation:
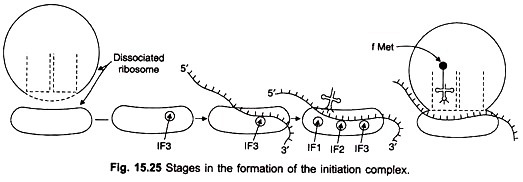
The first step in translation in bacteria involves the binding of three initiation factors, IF-1, IF-2 and IF-3 to the 30S small subunit of the ribosome (Figs. 15.24, 15.25). The mRNA and the N-formyl-methionyl tRNA join the complex. IF-2 recognises the initiator tRNA. IF-3 is released allowing a large 50S ribosomal subunit to associate with the complex.
The association triggers release of IF-1 and IF-2, resulting in the formation of a 70S initiation complex having mRNA and initiator tRNA bound to the ribosome. The complex is ready to begin peptide bond formation. Initiation in eukaryotes is more complicated and requires at least 10 proteins designated eIF-1 to elF-10 (eukaryotic initiation factors).
Step # 2. Initiation of Polypeptide Chains:
In E.coli and other prokaryotes the first amino acid in a polypeptide chain is methionine with a formyl group (CHO) attached to the free amino group. Initiation of protein synthesis therefore requires N-formyl-methionyl-tRNA (designated fMet-tRNAf), and not methionyl-tRNA. The transformylation takes place when a formyl group is transferred from N10-formyl-tetrahydrofolate to the α-amino group of methionyl-tRNA.
Thus the amino acid methionine in E. coli has tRNAs of two distinct species only one of which can initiate chain synthesis: methionyl tRNAMet and methionyl-tRNAfMet. Only the methionyl-tRNA of second species (tRNAfMet) can accept the N-formyl group and become formylated. Both species of tRNA have the same anticodon sequence UAC, but have slight difference in the remaining nucleotide sequence.
Formylation takes place only after the amino acid has become attached to the tRNA molecule, and is catalysed by the enzyme transformylase. The reaction is N10-Formyl-tetrahydrofolate + Met-tRNAf → tetrahydrofolate + fMet + tRNAf. The other species of tRNAfMet-tRNAMet cannot be formylated nor is recognised by transformylase.
The starting codon AUG for methionine is read both by tRNAfMet and by tRNAMet. The triplet GUG which codes for valine is read also by tRNAfMet but not by tRNAMet.
Thus both AUG and GUG are starting codons and can lead to the placement of formylmethionine in the first position of the polypeptide chain. When AUG is present in an internal position in mRNA, a methionine is inserted in an internal position in the chain; when GUG is present internally in mRNA it codes for valine.
It now appears likely that not only in bacteria but also in mitochondria of eukaryotic cells, fMet-tRNAf initiates protein synthesis.
Step # 3. Chain Elongation:
Elongation begins when the initiating aminocyl-tRNA is positioned on the peptidyl site (P) opposite the starting codon on mRNA and aminocyl site (A) is free.
Elongation is achieved in 3 steps as follows:
(a) Binding at Site A:
The aminoacyl-tRNA corresponding to the first triplet after the staring codon is bound to the aminoacyl site (A) in the following way. First the aminocyl tRNAx (x denotes any of the 20 amino acids) binds to a specific protein, an elongation factor (EF-T). Actually EF-T contains 2 subunits namely EF-T8 and EF-Tu.
Now EF-T combines with GTP to form an EF-Tu-GTP complex and EF-TS is released. The EF-Tu-GTP complex now binds the aminoacyl-tRNAx to form EF-Tu-GTP-ammoacyl-tRNAx complex.
This complex now binds to the ribosome in such a way that the aminoacyl-tRNAx is placed on site A, while the anticodon triplet becomes hydrogen bonded to its specific codon in mRNA. In eukaryotes the elongation factors are designaged EF-1 and EF-2 and they act differently.
(b) Peptide Bond Formation:
When the P site is occupied by the fMet-tRNAf and the A site by the aminoacyl-tRNAx, a peptide bond is formed between the amino group of the amino-acyl- tRNAx and the carboxyl carbon of the fMet-tRNA.
The enzyme peptidyl transferase present in the 50S subunit of the ribosome catalyses this reaction (Fig. 15.26). The result is a dipeptide attached to the tRNAx at site A; the tRNA at the P site has discharged its amino acid (fmet) and is itself empty, but is bound to P site.
(c) Translocation:
While the tRNA carrying the dipeptide is still bound to the A site opposite the mRNA codon of the newly added amino acid x, the ribosome moves to the next codon on mRNA. Simultaneously, the tRNA with dipeptide shifts from the A site to P site. This movement is called translocation and requires a specific protein, an elongation factor G(EF-G) and GTP.
The GTP first binds to EF-G to form a complex which binds to the ribosome. GTP hydrolyses to form GDP and P. The energy released during hydrolysis is utilised for a conformational change which causes the ribosome to move to the next codon on mRNA carrying the chain from the A site to the P site.
This brings the A site now opposite to a new codon, and a new aminoacyl-tRNA will now come and occupy the A site. After translocation EF-G dissociates from the ribosome.
Step # 4. Chain Termination:
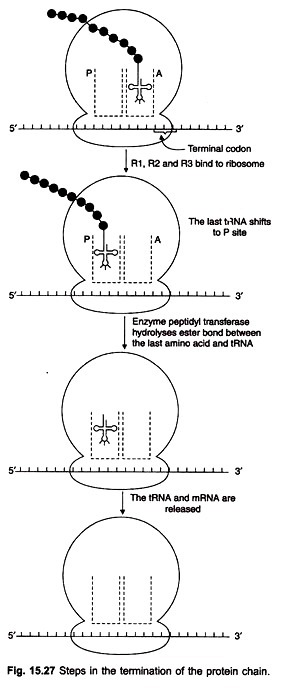
If there was no signal to stop chain growth, polypeptide chain would continue to elongate indefinitely. This is not so. Each one of 3 special termination codons in mRNA (UAA, UAG, UGA) can signal the termination of chain growth. These codons are read off by specific proteins, release factors R1 (responding to UAA and UAG) and R2 (responding to UAA and UGA).
When the termination codon is reached the polypeptide is still attached to the tRNA which is on the A site of the 50S subunit. For releasing the chain from the tRNA, release factors R1 and R2 are required (Fig. 15.27). They bind to the ribosome and cause the tRNA at A site to shift to the P site.
The enzyme peptidyl transferase hydrolyses the ester bond between the chain and the tRNA, aided by the bound releasing factors. When the polypeptide is released, the last tRNA and the mRNA are set free. This is then repeated by another ribosome which would associate with the mRNA as soon as the initiation point is free.