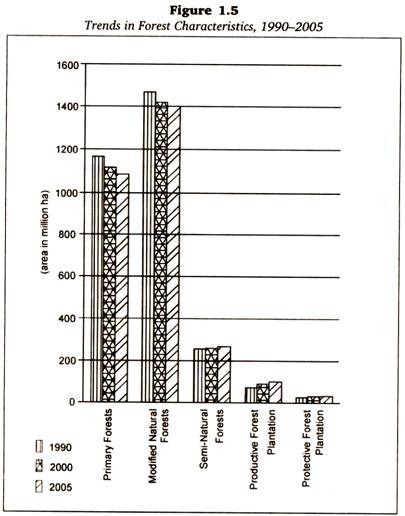
Read this article to learn about the production, collection and purification of recombinant proteins.
Gene Expression to Produce Proteins:
Several proteins have been produced by mammalian expression vectors. The yield of protein synthesis in some cases, is increased by inserting an intron between the promoter and the cloned gene. However, the reason for this is not clear.
Coordinated expression:
By coordinating the expression of a cloned gene and a selected marker, the production of recombinant protein can be increased. The coordinated expression of DHFR (marker) and a cloned gene is illustrated in Fig. 11.5. The DHFR gene is inserted close to a cloned gene and both of them are under the control of a single promoter and termination (polyadenylation) sequences. The DHFR gene is flanked by intron- removing sequence. As the genes are expressed, DHFR is synthesized by primary transcript while the recombinant protein is formed from spliced mRNA.
Expression of two cloned genes:
Some proteins are composed of two or more subunits. For example, hemoglobin is a tetramer with two copies of each subunit i.e., α2β2. Each subunit can be separately synthesized by the corresponding cloned gene, and they are then mixed to form the multimeric protein in vitro. This method is not very satisfactory since the in vitro assembly of multimeric proteins is not efficient. Techniques have been developed in recent years to produce two different proteins (i.e., subunits of a multimeric protein) in the same cell simultaneously.
Two-vector expression system:
Two expression vectors, each carrying a cloned gene for each subunit, are co-transfected into host cells (Fig. 11.6). These genes encode for the corresponding protein subunits. They then assemble to form a functional protein. The two-vector expression system has certain limitations — loss of one vector, overexpression of cloned gene in one of the vectors. This causes an imbalance in the formation and assembly of subunits.
Two-gene expression vector:
A single vector carrying two cloned genes is used to overcome the above problem. The two genes can be placed under the independent control of promoters and polyadenylation sequences (Fig. 11.7) to produce the assembled protein with two subunits. This method, however, does not ensure production of equal quantities of two subunits.
Dicistronic expression vector:
A dicistronic vector can be constructed with two cloned genes joined together to a small sequence of DNA that contains an internal ribosomal entry site (IRES). IRESs, found in mammalian viruses, allow a simultaneous transcription and translation to produce the assembled protein (Fig. 11.8). The transcription of gene-IRES-gene is under the control of a single promoter and termination signals. By using dicistronic expression vector, the synthesis of equal amounts of the protein subunits can be achieved.
Collection and Purification of Recombinant Proteins:
As the recombinant proteins are produced by the cloned genes, they start accumulating. The next task is to collect and purify the specific gene product i.e., the requisite protein. This is not an easy job since many a times the recombinant protein is foreign to the host cell which possesses an enzyme machinery to degrade the outside proteins.
Thus, human insulin produced in the bacterial host cells can be degraded by the proteases. This problem can be solved by using bacterial strains (e.g., E. coli) deficient in proteases. But there is a disadvantage since the proteases are defensive enzymes and hence the new strains lacking these enzymes are susceptible for easy destruction.
In an alternative approach, the recombinant proteins are fused with the native host proteins .The fusion proteins are resistant to protease activity. Sometimes, foreign proteins accumulate as aggregates in the host organism, minimizing the protease degradation. The problem with protein aggregates is that the biological activity may be lost while extracting the protein from the aggregates.
Export and secretion of recombinant proteins:
The yield of production of recombinant proteins is efficient if they are quickly exported and secreted into the environment (surrounding medium). Further, the recovery and purification of foreign proteins is easier from the exported proteins. Serious efforts have been made to develop methods for increasing the export of recombinant proteins.
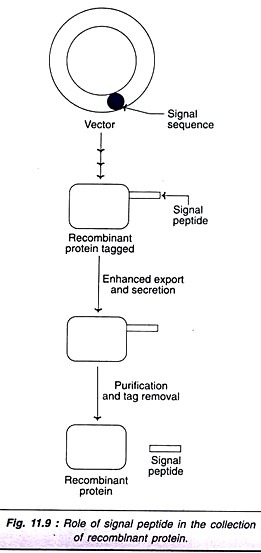
Some of the species of the bacterium, Bacillus subtilis normally secrete large quantities of extracellular proteins. A short DNA sequence, called signal sequence from such species is introduced into other B. subtilis. These bacteria produce recombinant DNA tagged with signal peptide, which promotes export and secretion (Fig. 11.9). The signal peptide can be removed after purification of foreign protein.
Signal sequence and signal peptide have been, in fact, effectively used for the production of recombinant insulin.
Purification of Recombinant Proteins:
There are several techniques in use for the purification of recombinant proteins from a mixture of secreted proteins.
Fusion proteins and purification:
The production of fusion protein has been described. Besides reducing the degradation, fusion proteins simplify the purification of recombinant proteins. A couple of purification techniques are briefly discussed hereunder.
Affinity tagging:
In this technique, a small DNA sequence encoding for a peptide (a short amino acid sequence) is ligated to the cloned gene. This peptide, in turn, has an affinity to bind to a compound or a macromolecule or even an element. The tagged amino acid sequence, which forms a part of the recombinant protein acts as an affinity tag or identification tag. The use of hexahistidine tag is briefly described in the next column.
The DNA sequence that encodes for six histidine residues (His6) is ligated to a cloned gene. The fusion protein (i.e., recombinant protein tagged with His6) can be isolated by passing the mixture of cell extract through a column packed with nickel-triacetic acid agarose beads.
The desired recombinant protein through its hexahistidine residues binds to the nickel ions (Ni2+). The rest of the proteins can be easily eluted from the column. The bound fusion proteins can be then eluted by lowering the pH of the buffer solution. Alternately, the fusion proteins can be selectively removed by binding of heaxahistidine tag to a competitor compound such as imidazole.
The next step is to remove the hexahistidine tag. This can be done by digestion with proteases which specifically act at the site of the tag. There is a need to remove hexahistidine residues only if the recombinant protein is being used as a therapeutic agent. For all other purposes, hexahistidine tag is acceptable, since it does not interfere in the normal structure and function of the proteins.
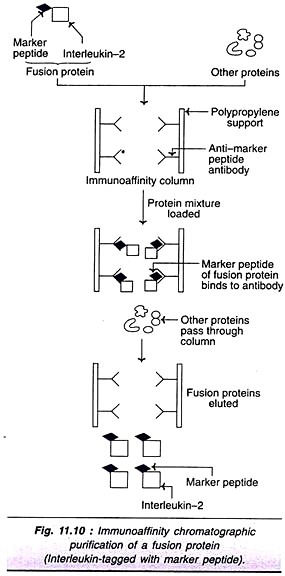
Immunoaffinity purification:
The immunoaffinity chromatographic purification technique of fusion proteins with special reference to human interleukin-2 is described. lnterleukin-2 gene joined to a small DNA sequence encoding a marker peptide (that synthesizes 8 amino acids, Asp-Tyr-Lys-Asp- Asp-Asp-Asp-Lys) produces a fusion protein in yeast (S. cerevisiae). The marker peptide has a dual function-reduces the degradation of interleukin-2, besides helping in its purification.
The fusion protein, interleukin-2 joined to a marker peptide can be purified by immunoaffinity chromatography (Fig. 11.10). The specific monoclonal antibodies (MAb) against the marker peptide are immobilized on a polypropylene support. These MAb serve as the ligand (anti- marker peptide antibodies) and selectively bind to fusion proteins tagged with marker peptide. However, the remaining proteins pass through the immunoaffinity column. The immuno-purified fusion proteins can be eluted later from the column.