Let us make an in-depth study of the proteins. After reading this article you will learn about: 1. Functions of Proteins 2. Structures of Proteins 3. Properties of Proteins and 4. Classification of Proteins.
Proteins are nitrogenous organic compounds of high molecular weight which play a vital or prime role in living organisms. They are made up of 20 standard a-amino acids.
Contents
Functions of Proteins:
The main functions of proteins in human body are:
1. They serve as body building units, e.g., muscle proteins.
2. They provide support and protection to various tissues, e.g., collagen and keratin.
3. All chemical reactions in the body are catalysed by proteinaceous enzymes, e.g., trypsin.
4. They transport various molecules and ions from one organ to the other, e.g., hemoglobin, serum albumin.
5. They store and provide nutrients, e.g., milk casein, ovalbumin.
6. They defend the body from harmful foreign organisms, e.g., immunoglobulin’s, fibrinogen.
7. They help to regulate cellular or physiological activity, e.g., hormones, viz., insulin, GH.
Structures of Proteins:
Primary Structure of Proteins:
Primary structure of proteins refers to the total number of amino acids and their sequence in that particular protein.
A fixed number of amino acids are arranged in a particular sequence. The sequence of amino acids in the protein determines its biological role. Different proteins have different sequences. Therefore, the study of total number and sequence of amino acids in a protein is the study of its primary structure.
Primary structure differentiates normal protein from abnormal one. Normal adult haemoglobin (HbA) is made up of 2 α-chains and 2 β-chains. Each α-chain has 141 amino acids and each β-chain has 146 amino acids arranged in a specific sequence. Any change in the sequence results in an abnormal haemoglobin. Like in sickle cell haemoglobin (HbS), the amino acid valine is present at the 6th position of P-chain instead of glutamic acid in the normal haemoglobin.
Secondary Structure of Proteins:
It refers to the twisting of the polypeptide chain into a helical form.
Three types of helical structures are found:
(a) Alpha helix
(b) Beta pleated and
(c) Reverse turn.
1. Alpha helix:
α means the first and the structure described below was the first among the helical structures to be discovered, hence known as alpha (α) helix.
The salient features of this structure are as under:
i. Here the polypeptide is twisted or coiled to form a right handed helical structure.
ii. The distance between each turn of the coil is 5.4 Å.
iii. There are 3.6 amino acids per turn.
iv. The ‘R’ groups are seen protruding out of the helix.
v. There are intra chain hydrogen bonding, wherein the hydrogen of —NH group combines with oxygen of -CO group of the 4th amino acid behind it. So every peptide group participates in hydrogen bonding.
vi. This type of structure is found in many proteins in combination with other structures. Pure a-helix structure is seen in hair protein, i.e., keratin.
2. Beta pleated:
β means the second and the structure described below was the second discovery after α helix.
The salient features of this structure are:
i. Here the chain is not helical but zigzag.
ii. The distance between each turn is 7 Å.
iii. Polypeptide chains are arranged side by side in the form of pleats.
iv. There is inter-chain hydrogen bonding between the chains and each peptide group participates in hydrogen bonding.
The chains are anti-parallel to each other.
3. Reverse turn:
Folds back on itself in reverse direction of the chain.
Tertiary Structure of Proteins:
The helical form of polypeptide folds into spherical, globular, ellipsoidal or other conformation, which is called the tertiary structure of proteins. This folding is necessary for the biological activity of the proteins. e.g., enzymes, immunoglobulin’s.
The tertiary conformation is maintained by four types of bonds:
1. Hydrogen bonds:
Formed between hydrogen and an electronegative atom like oxygen or nitrogen in the ‘R’ group of amino acids.
2. Ionic interactions:
Formed between acidic (glutamic and aspartic) and basic (arginine, lysine or histidine) amino acids.
3. Disulphide bonds:
This is a strong bond formed between the sulphahydryl groups of two cysteine amino acids. The resultant dimer structure formed is known as cystine (an amino acid found in proteins only and not in free form).
4. Hydrophobic interactions:
The ‘R’ groups of the hydrophobic amino acids aggregate together in the centre away from water, thereby developing a force of attraction between each “R” group and a force of repulsion from the water and these interactions are known as hydrophobic interactions.
Quaternary Structure of Proteins:
Quaternary structure is exhibited by oligomeric proteins.
Oligomeric proteins:
Are those which have two or more polypeptide chains.
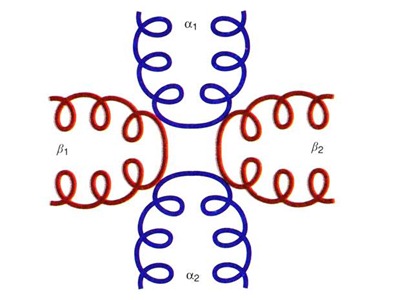
Quaternary structure refers to the type of arrangement of the polypeptides in an oligomeric protein. These polypeptides are held together by either hydrogen bonds, ionic bonds or Vander Waals’ forces, e.g., Hemoglobin has four polypeptide chains which are arranged in a particular fashion that is referred to the quaternary structure of hemoglobin.
The Quaternary structure of hemoglobin describes that it is made up of four polypeptide chains; two of which are α (α1 & α2) and the other two are β (β1 & β2). The two alpha chains are opposite to each other and adjacent to each β-chain. The α chains and the β chains are linked together by salt bridges.
Structure function relationship in proteins:
Hemoglobin plays a vital role in transport of oxygen from the lungs to the peripheral tissues and transport of carbon dioxide from the tissue to the lungs.
There are three types of normal hemoglobin with the following polypeptides:
(1) Adult hemoglobin (Hb A) has 2α2β chains.
(2) Foetal hemoglobin (Hb F) has 2α2γ chains.
(3) Minor adult hemoglobin (Hb A1) has 2α2δ chains.
The number of amino acids in α chains is 141 amino acids and the other chains, i.e., β, γ & δ chains have 146 amino acids. These chains are differentiated based upon the difference in the sequence of arrangement of the amino acids in the chains. The quaternary structure of hemoglobin creates a cavity in between the tetramer in which 2, 3, diphosphoglycerate (DPG or BPG) is present forming a salt bridge with the amino terminal of β-chain that stabilizes the hemoglobin thereby lowering the affinity to oxygen.
In the lungs, the partial pressure of oxygen is high which results in binding of O2 to one of the chains of Hb thereby rupturing the salt bridges between the four subunits. Subsequent oxygen binding (sigmoid curve of Hb-O9 association) is facilitated by rupture of the salt bridges altering secondary, tertiary and quaternary structures thus allowing rotation of one α/β subunit with respect to another α/β chain thereby compressing the tetramer and release of DPG. This results in increasing its affinity towards oxygen (the R state of Hb).
In the peripheral tissues, CO2 binds with the a-amino group of the amino terminal with its conversion from positive to negative charge which favours salt bridge formation between the polypeptide chains with return to the deoxy state (T-state), i.e., release of oxygen from Hb. Release of O2 from the Hb is also facilitated by binding of DPG to the tetramer.
When a person takes off on a flight, the aero plane slowly rises in altitude resulting in lowering of the O2 tension due to which oxygenation of Hb is not possible. Thus the person feels hypoxic, but the physiological mechanism of the body starts decreasing the production of DPG, due to which Hb can bind the oxygen even at lower pressure of oxygen.
Therefore, when the aero plane reaches the maximum altitude and stays stable, the person feels comfortable. When Hb reaches the tissues DPG level increases enhancing release of oxygen. Similarly, the above process reverses, when a person dives deep into the sea. The high O2 pressure results in increased production of DPG facilitating oxygenation of Hb in the lungs and deoxygenating in the peripheral tissues.
Properties of Proteins:
1. Denaturation:
Partial or complete unfolding of the native (natural) conformation of the polypeptide chain is known as denaturation. This is caused by heat, acids, alkalies, alcohol, acetone, urea, beta- mercaptoethanol.
2. Coagulation:
When proteins are denatured by heat, they form insoluble aggregates known as coagulum. All the proteins are not heat coagulable, only a few like the albumins, globulins are heat coagulable.
3. Isoelectric pH (pH1):
The pH at which a protein has equal number of positive and negative charges is known as isoelectric pH. When subjected to an electric field the proteins do not move either towards anode or cathode, hence this property is used to isolate proteins. The proteins become least soluble at pHI and get precipitated. The pHI of casein is 4.5 and at this pH the casein in milk curdles producing the curd.
4. Molecular Weights of Proteins:
The average molecular weight of an amino acid is taken to be 110. The total number of amino acids in a protein multiplied by 110 gives the approximate molecular weight of that protein. Different proteins have different amino acid composition and hence their molecular weights differ. The molecular weights of proteins range from 5000 to 109 Daltons. Experimentally the molecular weight can be determined by methods like gel filtration, PAGE, ultra centrifugation or viscosity measurements.
Classification of Proteins:
Proteins are classified based upon:
(1) Their solubility and
(2) Their structural complexity.
A. Classification Based upon Solubility:
On the basis of their solubility in water, proteins are classified into:
1. Fibrous proteins:
These are insoluble in water. They include the structural proteins. They have supportive function (e.g., collagen) and/or protective function (e.g., hair keratin and fibrin).
2. Globular proteins:
They are soluble in water. They include the functional proteins, e.g., enzymes, hemoglobin, etc.
B. Classification Based upon Structural Complexity:
On the basis of their structural complexity they are further divided into:
(1) Simple
(2) Conjugated and
(3) Derived proteins.
1. Simple proteins:
Proteins which are made up of amino acids only are known as simple proteins.
They are further sub-divided into:
(a) Albumins:
They are water soluble, heat coagulable and are precipitated on full saturation with ammonium sulphate, e.g., serum albumin, lactalbumin and ovalbumin.
(b) Globulins:
They are insoluble in water, but soluble in dilute salt solutions. They are heat coagulable and precipitate on half-saturation with ammonium sulphate, e.g., serum globulin and ovo-globulin.
(c) Glutelins:
They are insoluble in water and neutral solvents. Soluble in dilute acids and alkalies. They are coagulated by heat, e.g., glutelin of wheat.
(d) Prolamines:
Water insoluble but soluble in 70% alcohol, e.g., gliadin of wheat, proteins of corn, barley, etc.
(e) Histories:
Water soluble, basic in nature due to the presence of arginine and lysine, found in nucleus. They help in DNA packaging in the cell. They form the protein moiety of nucleoprotein.
(f) Protamine’s:
Water soluble, basic in nature, not-heat coagulable. Found in sperm cells, hence component of sperm nucleoprotein.
(g) Globin’s:
They are water soluble, non-heat coagulable. e.g., globin of haemoglobin.
(h) Albuminoids or scleroproteins:
Insoluble in all neutral solvents, dilute acids or alkalies, e.g., keratin of hair and proteins of bone and cartilage.
2. Conjugated proteins:
Proteins which are made up of amino acids and a non-amino acid/protein substance called the prosthetic group are known as conjugated proteins.
The various types of conjugated proteins are:
(a) Chromo proteins:
Here the non-protein part is a coloured compound in addition to the protein part. Ex. Haemoglobin has heme as the prosthetic group and cytochromes also have heme.
(b) Nucleoproteins:
These proteins are bound to nucleic acids, e.g., chromatin (histones + nucleic acids).
(c) Glycoproteins:
When a small amount of carbohydrate is attached to a protein it is known as glycoproteins, e.g., mucin of saliva. (Note: Glycoproteins have major amounts of protein and some amount of carbohydrates and proteoglycans contain major amounts of carbohydrates and little amount of proteins).
(d) Pbosphoprotein:
Phosphoric acid is present with the protein. Ex. Milk casein and egg yolk (vitellin).
(e) Lipoproteins:
Proteins in combination with lipids, e.g., LDL, HDL.
(f) Metalloproteins:
They contain metal ion in addition to the amino acids, e.g., hemoglobin (iron), ceruloplasmin (copper).
3. Derived proteins:
They are the proteins of low molecular weight produced from large molecular weight proteins by the action of heat, enzymes or chemical agents.
Proteins → Proteans → Proteoses → Peptones → Peptides → Amino acids