Let us make an in-depth study of the proteins. After reading this article you will learn about 1. Structure of the Proteins and 2. Classification of Proteins.
Proteins:
Proteins are organic nitrogenous compounds in which a large number of amino acids are joined together by peptide linkages to form long polypeptide chains. Peptide-linkage (—CONH—) is formed when amino group (—NH2) of one amino acid condenses with carboxylic group (—COOH) of another amino acid eliminating one molecule of water.
That end of the polypeptide chain where the —COOH group of the amino acid is not involved in peptide linkage is called as C-terminal end. The other end of the polypeptide chain with amino acid having free —NH2 group is called as N-terminal end.
Although there may be hundreds of amino acids in a single polypeptide chain but fundamentally there are only about 20 different types of amino acids that constitute proteins in plants (there may be repetition of amino acids continuously or at intervals in the polypeptide chain).
Because of their very large size the proteins are often called as gigantic molecules or macromolecules of the cells. Their molecular weight may range from few thousands to over a million (10 lakhs.)
Structure of the Proteins:
The structure of the proteins can be studied under the following heads:
(1) Primary Structure of the Proteins:
Specific sequence or the arrangement of amino acids in the polypeptide, chain constitutes the primary structure of the proteins (Fig. 9.25).
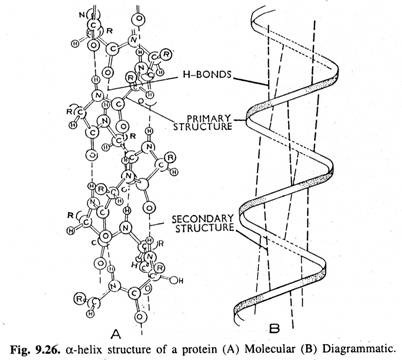
(2) Secondary Structure of the Proteins (or a-Helix Structure):
Polypeptide chain of the protein molecule is held in a coiled or helical shape by hydrogen bonds which are established in between the peptide linkages. The coiled or helical shape of polypeptide chain constitutes the α-helix or secondary structure of the protein (Fig. 9.26).
Although hydrogen bonds are very weak but when they are present in very large number all along the backbone of the polypeptide chain, they reinforce one another to stabilize the helical structure.
In a typical helical protein,
i. Each NH group (of peptide bond) is connected to a C=O group (of another peptide bond) by a H-bond at a distance equivalent to 3 amino acid residues.
ii. An α-helix or complete turn of a coil contains about 3.67 amino acid residues.
iii. The pitch of the helix is 5.4 Å (vertical distance along the axis from any point on the helix to a corresponding point directly above it is called as the pitch of the helix).
iv. Therefore, each amino acid is about 1.5 Å (5.4/3.6) distant from the next amino acid residue.
Besides a-helix structure, other types of secondary structures of proteins also occur. Among these, β-conformations (P-pleated sheets) are most common that are found in fibrous proteins called β-keratins. In β-pleated sheets, a number of polypeptide chains (which do not form helices) are cross linked with H-bonds in which amino acid to carboxyl terminal orientation may be in the same direction (parallel) or inverse (antiparallel).
Linus Pauling and Robert Corey are credited with doing pioneer work on peptide bond and organization of proteins. They even predicted the existence of secondary structures of proteins many years before the first complete structure of protein was elucidated.
(3) Tertiary Structure of the Proteins:
The coiled (α-helix) polypeptide chain is further folded in various ways. This folding which is very specific for a particular protein constitutes its tertiary structure and is determined by its primary structure (Fig. 9.27).
(Tertiary structure of the proteins is essential for biologically active proteins i.e., the enzymes. They are rendered useless (or are denatured) if their tertiary structure is lost).
The tertiary structure of the protein is stabilized by the following forces which have also been shown in Fig. 9.28.
i. H-bonds (other than those established between the peptide linkages).
ii. Di-sulphide (S-S) bonds.
iii. Ionic bonds or salt linkages.
iv. Steric Effects i.e., the interaction of non-polar side chains caused by the mutual repulsion of the solvent.
v. Van der Waals forces.
(All the molecules exert a week force of attraction upon one another due to mutual interaction of their electrons and nuclei. There is a electrostatic attraction between the electrons of one molecule and the nuclei of the other while on other hand, there is electrostatic repulsion of nuclei and electrons of the molecule by the nuclei and electrons of the other molecule respectively. The resultant week force of attraction between the two molecules is known as the Van der Waals attraction or Van der Waals force. The energy of such forces is about 1 k.cal/mole).
(4) Quaternary Structure of the Proteins:
Sometimes more than one polypeptide chains are associated together to form a relatively more stable super molecule of protein. This constitutes the quaternary structure of the protein.
For example, in blood haemoglobin there are four polypeptide chains or subunits that constitute the protein.
Quaternary structure is maintained by various forces like di-sulphide-linkages, H-bonds etc., between the different polypeptide chains of the protein.
Classification of Proteins:
On the basis of the nature of the products of hydrolysis by acids or proteolytic enzymes, the proteins are grouped under two categories:-
(A) Simple Proteins:
These proteins on hydrolysis yield only amino acids. On the basis of solubility properties simple proteins are classified as follows:-
1. Albumins:
Soluble in water and salt solutions.
2. Globulins:
Sparingly soluble in water but soluble in salt solutions.
3. Prolamins:
Soluble in 70-80% alcohol but insoluble in water and absolute alcohol.
4. Glutelins:
Insoluble in all the above solvents but soluble in acid or alkali.
5. Scleroproteins:
Insoluble in aqueous solvents (found in animals only).
(B) Conjugate Proteins:
These proteins on hydrolysis yield amino acids plus some non-amino acid part called as the prosthetic group.
Depending upon the nature of the prosthetic group associated with them, conjugate proteins are classified as follows:-
1. Nucleoproteins (or Histones):
These are associated with nucleic acids.
2. Glycoproteins:
These are associated with some carbohydrate.
3. Chromoproteins:
These proteins are associated with some colouring matter e.g., chlorophylls, carotenoids, phycobillins etc.
4. Lipo-proteins:
These are associated with some lipid or fatty substances. (They are chiefly found in cy- to membranes).
5. Iron-porphyrin Proteins:
These are associated with iron-porphyrin compounds e.g., the cytochromes.
6. Simple Metal Containing Proteins:
These proteins are associated with some metal directly e.g., ferredoxin where Fe atoms are directly attached to the protein molecule.
7. Flavoproteins:
These are associated with some flavin compound e.g., FAD (Flavin Adenine-Dinucleotide).