The following points highlight the top six microbial sources of enzymes. The sources are: 1. Production of Enzymes by Microbial Fermentation 2. Strain Improvement 3. Fermentation 4. Isolation and Purification 5. α-Amylase 6. Amyloglucosidase.
Microbial Source # 1. Production of Enzymes by Microbial Fermentation:
Enzymes are proteins, which catalyse specific biochemical reactions in a very efficient manner. Enzymes have been used for thousands of years as crude animal and plant preparations or as whole microorganisms, which were allowed to grow on substrates. The industrial production of enzymes dates back to 1894 when ‘fungal taka-diastase’ was marketed for pharmaceutical use.
Initially, enzymes were produced by solid-state fermentation, which is still used in Japan. But most current production processes are based on submerged cultures which are effectively aerated; the O2 requirement is generally as high as that for antibiotics. Most industrially important enzymes are extracellular, which makes their recovery from the broth relatively easier.
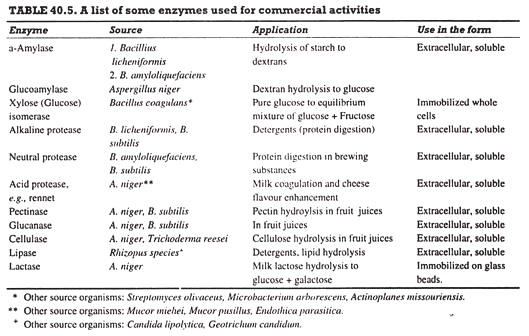
A number of enzymes are produced on large scale and used in commercial operations (Table 40.5). Of these, Bacillus protease, glucoamylase and amylase are produced in the largest quantities (Ca. 300 ton/yr of each). In addition, a large number of enzymes are produced on much smaller scales, and are mainly used for research (biochemical and medical investigations).
Microbial Source # 2. Strain Improvement:
Enzyme yield can be increased by careful selection of the organism, improvements in fermentation process, strain development, and refinement of downstream processing. The end use of the product and the conditions under which it is to be used will dictate the choice of organism and the product purity.
The basic strategies for primary screening and strain improvement will be the same. But the ultimate objective of strain improvement is to obtain regulatory mutants for the concerned enzyme so that its production is no more subject to the normal regulation. When immobilized cells are to be used, cell lysis must be absent and all the enzyme should be retained within the cells.
Microbial Source # 3. Fermentation:
Inoculum is developed in several stages as is the case with other fermentations. The organism may then be multiplied in one or more seed-tank stages. The medium used for these stages is rather rich to support good growth. The seed-tank culture is finally inoculated into the production stage fermenter (25,000-100,000).
The production medium is almost always complex and is devised to maximise yields of the enzyme. Appropriate measures must be employed to ensure contamination control. Biomass of the organism begins to increase early during fermentation, while enzyme activity shows significant increases about midway through the fermentation; the end of fermentation is signalled by cessation of enzyme synthesis.
Microbial Source # 4. Isolation and Purification:
Isolation and purification is done immediately after termination of fermentation in a manner that retains the enzyme activity. If the cells are to be used for immobilization, the biomass is isolated and treated to make it ready for use.
The extracellular enzymes are recovered directly from broth, while enzymes localized within cells are isolated by rupturing the cells. Enzyme purification is based on various techniques whose efficacy and cost differ widely; the process used will mainly depend on the purity needed and the cost that is acceptable.
Microbial Source # 5. α-Amylase:
This enzyme is an endohydrolase; it hydrolyzes starch into components, which have 3 or more linear α-1,4-glucan units. It stops hydrolysis when fragments with 2-6 glucose units remain; typically, such fragments contain an α-1.4-6 linked branch-point residue.
The end products of starch hydrolysis are dextrins, which are used as adhesives and thickening agents in prepared foods. α-Amylase must be used at high temperatures. The enzyme from Bacillus licheniformis can be used for prolonged periods at 95°C and for a brief period at 105-110°C.
The bacteria are grown on complex media based on maize or potato starch supplemented with soybean meal or corn steep liquor (medium has 20% dry matter). The fermentation is carried out for about 5 days, then the broth is chilled and the cells and solids are removed by flocculation.
The enzyme is extracellular, and is recovered from the broth; it is always stabilized with Ca2+ ions. Its applications are as follows dextrin production, first stage in glucose manufacture, in brewing and bakery, for removal of starch in textile manufacture, etc.
In starch hydrolysis, typically 40% starch slurry is heated to 105-110°C to which a thermostable enzyme is added along with Ca2+ salts (liquefaction). The partially hydrolysed starch is cooled to 90-100°C, more enzyme is added and incubated for 1-2 hr. The final slurry is reheated to about 120°C; acid may be added to inactivate the enzyme.
Microbial Source # 6. Amyloglucosidase:
Also called glucoamylase, this enzyme is an exohydrolase, and removes terminal glucose residues, one-by-one, from dextrins. This is produced by fungi, such as, Aspergillus or Rhizopus.
The strains used for enzyme production are regulatory mutants (enzyme synthesis is not repressed by free glucose), which are grown on α-amylase digested starch (20% w/v) medium. The fermentation lasts for 4-5 days at pH 4.5, and is N-limited. The enzyme is extracellular and is concentrated to about 5% active enzyme.
The dextrins obtained by α-amylase digestion of starch are further digested to glucose by glucoamylase. The α-amylase hydrolysate (30-40% sugars) is cooled to 65°C, pH is adjusted to 4, the enzyme is added, and the mixture is incubated for 2-3 days. At the end, the enzyme is inactivated by heat.