In this article we will discuss about the production of microbial biomass. Learn about the processes used for the production of biomass such as brewers’ yeast and Candida utilis yeast.
Traditional processes for the production of biomass such as the production of brewers’ yeast and Candida utilis yeast will be treated only in summary form. Newer processes will be described with somewhat more detail; and some proposed processes will be mentioned if they are of sufficient economic importance or academic interest.
Brewers’ Yeast:
During the fermentation of brewers’ wort there is a 3- to 8-fold multiplication of yeast cells. This yeast can be readily recovered by centrifuging, and a small portion is often used for inoculation of the next batch of unfermented wort. Of the excess yeast the largest portion is dried together with brewers’ spent grains and sold as feed.
In the United States a considerable portion, about 11.25 million kg (25 million lb) of yeast solids, is dried separately or used in the production of brewers’ yeast auto-lysate. This represents approximately 20% of the total available amount of brewers’ yeast.
Feed grade yeast is dried directly from the centrifuged yeast cream which contains about 10-15% yeast solids. Food grade yeast is debittered by an alkaline wash at pH 8 or above followed by washing with water. It is then dried by spray drying or occasionally by atmospheric drum drying. It is used as an ingredient in fabricated foods or sold as a dietary supplement.
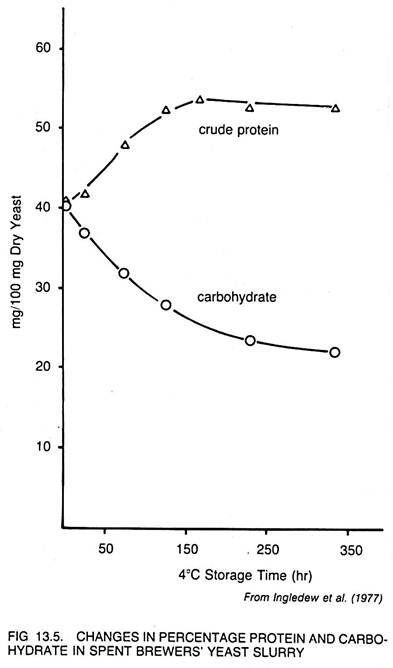
Processes for the production of brewers’ yeast have been described by Quittenton (1966) and Hunt (1969). Brewers’ yeast in liquid form which does not meet a minimum content of 40% of crude protein may be stored at 4°C until endogenous metabolism of reserve carbohydrates leads to a higher protein level in the yeast. While the total amount of protein does not change during this storage, its relative percentage of cell solids increases as shown in Fig. 13.5.
Distillers’ and Wine Yeast:
In neither of these industries is it feasible to separate the considerable amounts of yeast which have grown during the fermentation. In distilleries, the yeast is recovered in the spent distillers’ grains and sold to the feed industry. In the wine industry, the yeast is discarded with the lees.
Bakers’ Yeast:
Inactive dried bakers’ yeast is too expensive for use in the feed industry. However, it is used as a nutritional supplement. Since this is primary grown yeast, i.e., yeast grown specifically for sale as such, it is possible to increase the crude protein content to 50-55% and to induce high levels of thiamin and nicotinic acid during the growth process.
Other Carbohydrate Substrates:
Sulfite Liquor:
A widely available carbohydrate substrate is spent sulfite liquor which is a by-product of paper pulp mill operations. Liquor from hardwoods contains about 2-3% of fermentable sugars of which about 80% are pentoses and 20% hexoses. Liquor from softwoods contains about 80% of the sugars in the form of hexoses and 20% as pentoses.
Romantschuck (1975) gives the following breakdown for the various sugars in spruce sulfite liquor- mannose 50%, glucose 12%, galactose 12%, xylose 20%, arabinose 4%. In addition, sulfite liquor contains appreciable amounts of acetic, galacturonic and formic acids.
Candida utilis, which assimilates pentoses, hexoses, and many organic acids, has been produced commercially for several decades. It is purprising that this fermentation has not been treated more extensively in the literature. The basic papers describing current operations have been published by Inskeep et al. (1961) and Butschek and Krause (1962), and the process has been reviewed by Reed and Peppier (1973). The aerobic process is carried out continuously with a dilution rate of 0.27 to 0.3, at a pH of 4.5 and a temperature of 32°C. A nitrogen source (ammonia), phosphate, and potassium have to be supplied. Addition of biotin is not required. The effect of dilution rate on sugar utilization and yield has been investigated by Lorenz et al. (1967).
C. utilis is recovered by centrifuging. Minimal washing results in feed yeast which still contains about 10% lignosulfonic acids. Additional washing results in food grade yeast free from lignosulfonic acids. In the United States a major portion of the C. utilis produced on sulfite liquor is used in fabricated foods. One of the more recent plant installations has been described by Anderson et al. (1974).
C. utilis for use in feeds is also grown extensively in Eastern Europe as well as in Cuba and Taiwan. In the latter two countries molasses is the substrate.
In Finland, sulfite liquor is used in the production of a microfungus (probably Paecilomyces varioti) for use as a feed ingredient. The gross chemical composition of the dried mycelium, its RNA content, and its amino acid composition are fairly similar to that of C. utilis. The process is carried out on a continuous basis with a retention time of 4.5 to 5 hr. The process has an advantage in that the fungal mass can be recovered with a rotary filter instead of a centrifuge. The process is known as the “Pekilo” process.
Additional process details have been described by Romantschuk and Lehtomaki (1978), and the reduction of the NA content of the “Pekilo protein” by Viikari and Linko (1977). Two 360 m3 fermentors (agitated) are in operation. The yield of biomass is 55% based on the consumption of reducing materials; and the productivity is 2.7-2.8 g of fungal solids per liter per hr.
Whey:
Cheese whey is an excellent substrate for the production of biomass. It contains about 5% lactose, 0.8% protein, 0.7% mineral matter, and from 0.2 to 0.6% lactic acid. Lactose is assimilated by “dairy” yeasts. Kluyveromyces fragilis and K. lactis are the species used. The whey proteins may be separated by ultrafiltration prior to the fermentation; or they may be partially removed by acid/heat coagulation.
The fermentation may be carried out with incremental feeding of the whey or whey concentrate or as a continuous process. A yeast inoculum of 1 x 109 cells per ml, a pH of 4.5, and a temperature of 30°C are quite satisfactory. For the continuous fermentation a dilution rate of 0.125/hr can be maintained. Almost all authors describing the growth of dairy yeasts on whey supplement the medium with yeast extract.
The principal purpose seems to be supplementation with biotin. The fermentation may be carried out aerobically for the production of yeast biomass or with minimum aeration for the production of ethanol. If the entire content of the fermentor is dried, the end product will be a feed grade material which contains yeast, residual whey proteins, fairly high ash content, and some lactic acid. If the yeast cells are separated by centrifuging and washed, the dried end product is a food grade material with a crude protein content of45 – 55% and a PER of 2.26.
The process gives a yield of about 45-55% of yeast solids based on lactose consumed. Addition of whey can be regulated on the basis of an automatic assay of lactose in the fermentor liquid. An economic analysis has been prepared by Pace and Goldstein (1975) based on an earlier process by Wasserman et al. (1961).
A 2-step fermentation process for the production of biomass has been described by German workers. Starting with whey or whey ultra-filtrates, they converted lactose to lactic acid by an anaerobic fermentation. This was followed by an aerobic fermentation with a lactic acid assimilating, thermophilic yeast.
A similar approach has been used by Skupin et al. (1977), who fermented whey anaerobically with Propionibac- terium shermanii or two other species of propionibacteria, followed by an aerobic fermentation with K. fragilis. The whey medium was fortified with 5, 6-dimethylbenzimidazole, a precursor of vitamin B12. This results in the production of propionibacteria with high levels of vitamin B12. These bacteria also contribute a higher level of sulfur-containing amino acids to the biomass.
A feed product may be produced from whey by fermentation with lacto- bacilli and neutralization with ammonia which results in the formation of ammonium lactate. The concentrated fermentor liquor is referred to as fermented, ammoniated condensed whey. In this product 70% of the nitro-, gen is derived from ammonium lactate, 17% from whey proteins, and 8% from the cells of L. bulgaricus.
Petrochemical Substrates:
The petrochemical substrates that have been considered or used for the production of biomass are n-alkanes, alkane-containing gas oils, methane, methanol, and ethanol. Commercial work has progressed furthest with alkane fractions, although some pilot plant or demonstration plants have been closed, and production start-up in other plants has been blocked by legal procedures.
N-Alkanes and Gas Oil:
The microorganisms most suitable for growth on n-alkanes are yeasts of the genus Candida. Generally bacilli do not grow on alkanes or they grow only weakly, although some are able to oxidize alkanes in the presence of other carbon sources.
The use of water-insoluble alkanes as substrate adds an extra phase to the fermentor content; that is, there are now 4 phases- the liquid alkane phases, the aqueous phase, the gas phase, and the cells themselves. Aside from the carbon source, the nutrients added are those well-known from the carbohydrate fermentation, namely, ammonia, phosphoric acid, K, Mg, Mn, Zn, and Fe.
Operation is usually on a continuous basis at a pH at or below 4 and at a temperature of 30°-32°C. The process developed by British Petroleum and practiced at its Grangemouth plant (3600 MT or 4000 tons/year) was operated aseptically and oxygen transfer was achieved with the aid of mechanical agitation. Other companies have developed processes operating non-aseptically and with the use of simple air lift fermentors (Gulf, Kanegafuchi, Liquichimica).
The alkane based processes have been described by Cooper et al. (1975), Kanazawa (1975), Laine and Chaffaut (1975), Knecht et al. (1977), and Allison (1975), among others. There are some fundamental differences between fermentations based on carbohydrates and those based on hydrocarbons.
For hydrocarbons the size of the hydrocarbon droplets is critical since growth of the yeast cells takes place through direct contact of the cell wall and the surface of the alkane droplets (for tridecane and higher alkanes). Oxygen requirements are higher, and approximately 2 g of O2 are required for each g of yeast solids grown on alkanes. The heat evolved during the fermentation is, therefore, proportionately high, approaching 0.11 kcal per mMol O2 consumed.
Yields are high in comparison with carbohydrate substrates. For n-paraffins they are between 0.95 and 1.15 g of cell solids per g of substrate. Productivities of up to 4 g/liter/hr can be achieved but it is not economical to operate at that rate since energy requirements increase disproportionately at productivities exceeding 2.5 g/liter/hr.
Alkane grown yeasts can be recovered by simple centrifuging. After washing, the cell mass can be spray dried resulting in a light tan powder of about 6% moisture. The gross chemical composition is 9.5% N, 1.6% P, 6% ash, and 8-10% lipids (after acid hydrolysis). The species most widely used is C. lipolytica. Knecht et al. (1977) more properly refer to this yeast which has a sexual cycle as Endomycopsis lipolytica.
Yeast grown on gas oil has to be extracted with solvents after recovery to remove adhering hydrocarbons. This results also in the extraction of some lipids from the cells and accounts for the relatively higher protein content of cells grown on gas oil. The earlier literature on the production of yeasts from hydrocarbons has been reviewed by Laine et al. (1976), Johnson (1967), Humphrey (1970), Fiechter (1967), and Gounelle de Pontanel (1972).
Methane:
The use of methane gas as substrate poses some extraordinary problems. Methane oxidizing organisms such as Methylococcus capsulatus are readily inhibited by small concentrations of extracellular products of metabolism. For instance, methanol inhibits such organisms strongly. This requires the removal of methanol from the fermentation medium as soon as it is formed.
While this can be achieved by continuous dialysis it is not a practical solution. An alternate solution is the use of mixed cultures of methane oxidizing organisms with organisms assimilating methanol, a procedure which is designed to remove the inhibiting material as fast as it is formed.
Methanol:
Both methanol and ethanol have some advantages in comparison with paraffins and methane. They are water soluble and they are commercially available in relatively pure form. Methanol is assimilated by several genera of yeasts and bacteria.
Sahm and Wagner (1975) list the methanol-using organisms as follows:
Foo (1978) dealt with the pathways of methanol formation by obligate and facultative methylotrophic organisms. The substrates for obligate methylotrophs include methane, methanol, formic acid, formamide, carbon monoxide, dimethyl ether, dimethyl amine, and trimethyl amine.
The methanol metabolism of bacteria and yeasts has been reviewed by Sahm and Wagner (1975) and Schlanderer et al. (1975). Prave and Sukatsch (1975) grew a strain of Pseudomonas at 37°C and at a pH of 6.65 and obtained a specific growth rate of 0.2/hr with a cell concentration of 18.9 g/liter and a yield of 41.5% based on methanol.
Dostalek and Molin (1975) used a strain of Methylomonas methanolica and achieved a maximum specific growth rate of 0.53/hr and a yield of 48%. They investigated the interrelationship among several variables (specific growth rate, temperature, yield, and productivity). Figure 13.7 shows the effect of temperature on productivity.
The process developed by Imperial Chemical Industries which is nearing – the commercial stage has been described by Littlehailes (1975) and Gow et al. (1975). The organism, Pseudomonas methylotropha, is a Gram-negative rod, catalase positive, and shows oxidative metabolism. Its optimum growth temperature is between 34° and 37°C and its optimum pH range is from 6.5 to 6.9. It could be grown at a specific growth rate of 0.5 per hr. A carbon conversion of 62% could be achieved and a cell concentration of 30 g/liter.
The continuous aerobic fermentation is carried out under aseptic conditions in an air lift fermentor which is pressurized and which has an external loop. The crude protein content of the microbial mass is 85% and the true protein content 64%. A demonstration plant producing a strain of Methylomonas clara was placed in operation by Hoechst Germany in 1978.
The isolation of methanol utilizing strains of yeasts has been described by Oki et al. (1972). Yeasts generally give lower yields than bacteria, namely 29 to 45% based on the weight of methanol. Specific growth rates for yeasts are also lower. Cooney and Makiguchi (1977) and Cooney and Levine (1975) have reported on their extensive work with Hansenula polymorpha.
The authors have addressed the problem of selecting the most economical conditions for biomass production in an exemplary manner. Figure 13.8 shows the effect of dilution rate and the Resulting methanol concentration of the medium on dry cell weight and productivity.
The authors conclude that “it is essential to give a strong bias to the effect of dilution rate on cell yield in
the selection of an optimum dilution rate. Thus the selection of an economically optimum dilution rate is comprised of several factors but, predominantly, it is the effect of dilution rate on cell yield which determines the selection.”
Ethanol:
The following genera of yeasts and bacteria have been considered for the production of biomass from ethanol:
Yeasts- Candida, Debaryomyces, Eridomycopsis, Hansenula, Mycoderma, Pichia, Rhodotorula, Saccharomyces
Bacteria- Acetobacter, Acinetobacter, Arthrobacter, Bacillus, Brevi- bacterium, Corynebacterium, Hyphomicrobium, Nocardia, Pseudomonas
In addition some edible mushrooms have been grown on ethanol, such as Lentinas, Pleurotus, and Schizophillum. In the United States, C. utilis is produced commercially on ethanol. The process uses a continuous fermentation in an agitated, aerated vessel of special construction. Details have been described by Ridgeway et al. (1975). Demonstration plants have been built in Japan for the production of C. ethanothermophilum and in Czechoslovakia for the production of unspecified yeast.
The assimilation of ethanol by bakers’ yeast has been known for a long time. Ethanol produced during oxygen-limited fermentations by S. cerevisiae is assimilated during later stages of yeast growth. However, ethanol assimilation is rather inefficient and it is not practical to grow bakers’ yeast on ethanol.
Japanese workers have also emphasized the need for a substrate which avoids the alleged toxicity problems with hydrocarbon media. Masuda (1974) and Masuda et al. (1976) have utilized the thermophilic yeast, C. ethanothermophilum. The yeast is grown at a temperature of 40°C, which reduces the cost of cooling considerably, at a pH of 3.5, and with a specific growth rate of 0.45/hr. The fermentation is carried out in an air lift type fermentor at a cell solids concentration of 20 g/liter.
A bacterial culture, Acinetobacter calcoaceticus, has been used by Laskin (1977A) for biomass production. The optimum pH for growth was between 6.5 and 7.5 and the optimum temperature between 32° and 35°C. The fermentation has to be carried out under ethanol-limiting conditions and high growth rates in order to prevent the accumulation of acetic acid and aldehyde which inhibit the fermentation.
This is also desirable to maximize the yield coefficient. Figure 13.9 shows the protein yield coefficient as a function of specific growth rate. The variation of the yield coefficient with growth rate indicates fairly high requirement for energy for maintenance metabolism. This was calculated as 0.11 g of ethanol per g of biomass per hr.
Cellulose arid Agricultural Waste:
The term “waste” in the above subtitle cannot be used with precision. A substrate used for the purpose of producing biomass ceases to be “waste.” In that sense molasses or whey cannot be considered waste when it is used for an industrial fermentation. Another difficulty arises from the fact that it is not always simple to distinguish between fermentation processes designed for the disposal of waste and those designed to yield a salable biomass. Only those processes will be discussed which are clearly intended for the production of microbial biomass-and the commercial sale of that biomass.
The materials which are potentially suitable for the production of biomass can be classified into timber, wood residues, and wood pulp, agricultural residues including feedlot waste, and solid waste from food processing operations. Almost all of these materials contain cellulose as the principal carbon source. Price, availability, and transportation problems differ greatly for the various substrates.
However, one problem is common to all of them. This problem is the difficulty of converting cellulose or other polymeric carbohydrates such as xylan to fermentable sugars. Cellulose is well digested by some microorganisms, particularly by some wood-rotting fungi but the “low growth rates of cultures on untreated cellulosics have made economically feasible biomass production from cellulose difficult to envision”.
Cellulolytic enzymes isolated from wood-rotting fungi have a low rate of activity, and there is little correlation between the efficiency of fungal attack on cellulose fiber and that of a cell-free extract of the fungi.
It is generally assumed that the principal obstacle to efficient enzymatic hydrolysis of cellulosic materials is the degree of crystallinity of cellulose and lignification. Bellamy (1975) stated emphatically that the “effective and rapid utilization of lignin and the cellulose lignin complex is the major obstacle to economic recycling of cellulosic waste.”
The amount of cellulose in different raw materials varies greatly from 90% in cotton fibers to 15-20% in leaves. Hardwood stems contain 40-50% cellulose, 20-40% hemicellulose, and 18-25% lignin; softwood stems contain 45-50% cellulose, 25-35% hemicellulose, and 25-35% lignin. Grasses such as bamboo, palms, wheat, rice, sugar cane, etc., contain 25-40% cellulose, 25-50% hemicellulose, and 10-30% lignin.
Newsprint generally contains the same percentages as the woods from which it was made. Animal manure contains relatively more lignin than other sources, presumably because of the digestion of cellulosic material in the rumen. Susceptibility of the various raw materials to enzymatic attack differs greatly. This has led to a broad search for pre-treatment methods of the substrate and for suitable microorganisms.
There are basically two approaches which can be used for the production of biomass on cellulosic materials. The first is the hydrolysis of cellulose by acid or enzymes followed by the growth of microorganisms on the formed glucose. The second is the growth of fungi which hydrolyze cellulose and assimilate the sugars directly.
Most of the work on acid hydrolysis of cellulose has been done in Eastern Europe and the literature on the process is not extensive. Earlier processes of acid hydrolysis had the inherent disadvantages of poor yields due to the formation of sugar reversion products and the need to dispose of sizeable quantities of salts derived from the added hydrochloric or sulfuric acid and alkali.
Recently the reaction of dry sawdust with 33% HCl and dry HCl has been suggested with a predicted yield of 95% glucose after a 90 min treatment. Grethlein (1978) found acid hydrolysis of newsprint more economical than enzymatic hydrolysis. Using a plug flow reactor for hydrolysis with 1% sulfuric acid, he found optimum conditions for a residence time of 0.19 min at a temperature of 230°C. One part of glucan yielded 0.55 parts of glucose, 0.24 parts of decomposed glucose, and 0.21 parts of unreacted glucan.
The use of concentrated H2SO4 as a solvent for cellulose has been emphasized recently. This can later be diluted and used as a catalyst for cellulose hydrolysis.
Enzymatic conversion of cellulose has been treated extensively in a recent symposium. Major obstacles to fast and efficient enzymatic hydrolysis have been the inaccessibility of cellulose due to poor penetration of the enzyme and the inhibition of cellulase activity by cellobiose.
Yields of wood sugars can be greatly increased by pre-treatment of the cellulose- chemically by treatment with alkali, amines, or ammonia or mechanically by comminution, preferably by ball milling. Working with newsprint as raw material, Wilke et al. (1976) have arrived at the most attractive cost projections. These were based on 50% conversion of cellulose with the cellulase of Trichoderma viride for 40 hr at 45°C with recycling of the sugar enzyme solution to agitate; concrete digesters.
The inhibition of cellulase by cellobiose may be overcome by diffusion of the formed cellobiose through membranes during hydrolysis. Alternately, the sugars formed during enzymatic hydrolysis may be removed through concurrent growth of a microorganism or through concurrent production of ethanol by fermenting microorganisms.
Meyers (1978) added C. utilis during the saccharification of cellulose by T. viride cellulase. In this coupled saccharification-fermentation process the rate of saccharification was about the same as in the absence of the yeast but it proceeded for a longer time. Ultimately, 20% more saccharification was achieved as shown in Fig. 13.10.
The process can also be carried out with S. cerevisiae. The principle is the same as that traditionally used in the production of distilled beverages where starch hydrolysis by malt enzymes proceeds simultaneously with the fermentation of the formed maltose by distillers’ yeast.
Direct cultivation of microorganisms on cellulose seems to promise the best results with thermophilic organisms. Since direct growth of microorganisms on cellulose is slow, the loss of substrate due to the maintenance metabolism of the organism is rather high. Use of thermophiles partially overcomes this obstacle.
Humphrey etal. (1977) worked with pure cellulose substrates (Avicel) and obtained yields of up to 45 g cell solids per 100 g of cellulose utilized and growth rates of 0.45/hr with a Thermoactinomyces species. Some work with other thermophiles on mixed agricultural wastes is listed below.
Dunlap (1975) used mesophilic organisms, a Cellulomonas species, and Alcaligenes faecalis with bagasse as substrate. The waste material required pre-treatment by swelling in dilute alkali. Cellulomonas has also been used in the process developed by Louisiana State University.
A great amount of work has been done with agricultural waste and food processing waste in various industries. Only a few examples can be given. Gregory et al. (1976) grew Aspergillus fumigatus on cassava at a pH of 3.5 and a temperature of 45°-50°C, obtaining yields of cell solids of 50%.
Macris and Kokke (1977) used the extract of carob bean pods to grow Fusarium moniliforme. Ladisch et al. (1977) and Church et al. (1977) worked with residues of the corn milling industry using fungi such as T. viride and Gliocladium deliquescens for conversion to biomass. Chahal et al. (1977) grew Chaetomium cellulolyticum on wheat straw.
Effluents from coffee plantations are good media for the growth of a Verticillium species at 33°C and at a low pH of 3.5. Sanchez-Marroquin (1977) used mixed cultures of yeast and bacteria for the production of biomass on agave juice. Tanner and Hussain (1977) hydrolyzed kudzu starch with a-amylase and grew strains of S. cerevisiae on the hydrolysate.
By-products of the malting and brewing industry have been considered as substrates by Pomeranz (1976) and Shannon and Stevenson (1975). A general review of earlier work with microfungi growing on carbohydrate wastes was published by Anderson et al. (1975).
It should be noted that the easily hydrolyzed starchy substrates can be readily fermented by yeasts of the Saccharomyces and Candida genera. Carbohydrates more resistant to enzymatic hydrolysis and containing pentosans, cellulose, or more complex polymers appear to be more suited for direct attack by fungi.
Secondly, it appears that much of the work reported aims at the reduction of the BOD of effluents rather than at the production of biomass per se. For instance, Fig. 13.11 shows the growth of C. utilis on sauerkraut brine and the concomitant reduction in BOD.
Feed lot waste consisting largely of cattle manure contains much non-protein nitrogen, but a considerable part of its nitrogenous constituents is protein of microbial origin resulting from the growth of rumen organisms. In view of the variability of the substrate it is not surprising that various authors report widely differing values for this fraction. Morrison et al. (1977) found that 45% of the protein of sieve-fractionated manure is of microbial origin. They describe a procedure for recovery of a protein fraction by extraction with 0.1 N NaOH followed by ammonium sulfate precipitation.
The use of cattle manure as a substrate has been considered by several research workers. It may be less attractive than other agricultural wastes because it is already partly digested by microbes, and transportation is an obvious problem. The latter problem can be minimized by building fermentation facilities on the site of the feedlots. A process for the anaerobic fermentation of feedlot waste has recently been put into operation. It generates methane as the principal end product, and the residual fermentation sludge is used as cattle feed.
Reddy and Erdman (1977) studied the liquid extract of feedlot waste as a potential substrate for fermentation. The waste, including urine, straw, wasted feed particles, etc., was slurried and filtered. The filtrate fraction contained 3.0 to 5.2% nitrogen, 35% ash, 3% cellulose, 7.0% lignin, and 6.0% non-cellulose carbohydrate on a total solids basis.
It was not practical to ferment this filtrate without the addition of other agricultural wastes high in carbohydrate content. But the additives actually used in the experimental work, such as whey, molasses, cornstarch, or potato starch, are probably not available for such supplementation in a practical manner. The fermentation of the liquid extract of feed lot waste with some of the mentioned additives proceeded best with the natural flora and less efficiently with added L. bulgaricus cultures or cultures of rumen organisms.
These are wastes from potato processing plants or from the wet milling of corn. One of these processes is based on the hydrolysis of starch by Endomycopsis fibuliger followed by growth of C. utilis. This “Symba” process is practiced in the Scandinavian countries.
Growth of the two species of yeasts indicates a symbiotic relationship. Because of the faster growth of C. utilis, the percentage of E. fibuliger cell solids is only 4% of the harvested biomass. The process has been described by Jarl (1969) and Skogman (1976).
In the United States a system for treating waste water from potato processing operations is designed mainly for the reduction of BOD but biomass becomes available as a by-product for use as a feed.
Details of this process have not been published. But a process for growing lactobacilli on acid hydrolyzed starch from potato processing waste has been described. The principal end product is ammonium lactate resulting from the neutralization of lactic acid with NH3. It is an excellent rumen feed.
Photosynthetic Bacteria:
Bacterial photosynthetic processes use molecular hydrogen, reduced sulfur compounds, such as H2S, or some organic compounds as electron donors. Water is not used as electron donor which means that oxygen is not liberated as the result of bacterial photosynthesis. The process is basically anaerobic. Table 13.8 shows the photosynthetic reactions of plants and bacteria in a schematic way. Using one species of non-sulfur purple bacteria, Shipman etal. (1977) grew Rhodopseudomonas gelatinosa on an infusion of wheat bran with suitable sunlight or incandescent illumination at a pH of 7 to 7.2 and a temperature of 37°C. Retention time in the fermentor was about 24 hr.
Chemolithotrophic Microbes:
Lafferty et al. (1975) experimented with aerobic organisms which can use molecular hydrogen as electron donor. These belong to the genera Hydrogenomas and Alcaligenes. Their rate of growth surpasses that of the phototrophic organisms. These mesophilic organisms grow well between 28° and 35°C and at a pH between 6.8 and 7.2.
Hydrogen, oxygen, and carbon dioxide (as the carbon source) have to be supplied as well as nitrogen in the form of ammonia, nitrates, or urea. The need to supply the organisms with an explosive gas mixture, and the formation of poly-β-hydroxybutyric acid as an energy depot of the cell is disadvantages. The formation of poly-β- hydroxybutyric acid can be blocked by genetic manipulation.
Algae:
Among phototrophic organisms, algae have been investigated widely as sources of biomass for food or feed. It is interesting to note that information on processes is meager in contrast to fairly extensive work on the feeding of algae to humans and animals. The use of algae as part of the human diet by the Aztecs of Central America and the natives of the Lake Chad area in Africa has been well established, but quantitative data on dietary intakes are lacking.
Algae use carbon dioxide as the carbon source. They derive energy from sunlight or artificial light in the range of 700 nm by means of the chlorophyll of the cells. Since algae grow abundantly on stagnant ponds, attempts to use these microbes as food or feed are a borderline case between traditional agriculture and modern microbial biomass production. From a practical point of view algal cultures should be carried out between the latitudes of 35°N and 35°S from the equator. Soeder and Mohn (1975) distinguish between the so-called clean processes with well- defined media and the waste water processes.
Clement (1975) described the cultivation of Spirulina in a semi-natural culture basin of Sosa Texcoco, South America, in cooperation with the Institut Francais du Petrole. The culture medium contains mineral salts and a certain amount of sodium carbonate and bicarbonate.
Optimum growth rates are obtained at a pH between 8.5 and 11 and at a temperature of 32°C. The mass transfer coefficient (KL) for CO2 absorption by suspensions of Scenedesmus quadricauda has been investigated by Livansky et al. (1973). They found it to be independent of the chemical reactivity of CO2 in the algal suspension.
The algae which have been considered for biomass production belong principally to the genera Chlorella, Spirulina, and Scenedesmus. The cell walls of algae interfere with full utilization of the protein, and, therefore, the results of feeding trials depend greatly on the treatment of the biomass after harvest. For untreated Scenedesmus or Chlorella digestibility was only 25%.
Spray drying, air drying, vacuum drying, and freeze drying improved the digestibility, but the best results, 70-80% digestibility, were obtained with drum drying or simple boiling of the biomass for 6-8 min. The feeding of algae to humans has been recently reviewed by Pabst (1975), Feldheim (1975), Frahm and Lembke (1975), and Pokrovsky (1975).
Secondary Processing of Microbial Biomass:
Secondary processing includes the recovery of biomass from the fermentor, concentration, and drying, as well as other processes designed to sterilize the material or to fractionate it. Recovery of yeasts is generally done by centrifuging of the fermentor liquid resulting in a product with 10-20% solids content.
This material may be washed with water once or several times followed by recentrifuging. In the case of paraffin grown yeast, a surfactant is added to remove traces of paraffins; however, for yeasts grown on gas oil a solvent extraction step must be included. Bacteria are more difficult to harvest because of their smaller size and lower density.
The rate of centrifugal separation is a function of the difference in density between the cells and the liquid medium, and it varies also with the square of the diameter of the cell. For algae growing on the surface of ponds, simple skimming is the most economical method of harvest.
Drying has been done traditionally on atmospheric drum dryers of the single or double drum type. Recent installations favor spray drying. An intermediate evaporation step is rarely required since centrifuging to 15 to 20% solids concentration of cell solids can be carried out.
Besides, yeast slurries such as bakers’ yeast or brewers’ yeast cannot be pumped well once the solids content exceeds 22-23%. Labuza et al. (1972A,B) found that below 10% solids the slurry showed Newtonian characteristics with viscosities below 4-5 centipoises.
For both drum drying and spray drying it is desirable to heat the yeast slurries prior to the drying step in order to kill contaminants and to reduce the number of viable cells of the biomass. For spray drying Labuza et al. (1972B) reported that in the range from 21° to 46°C for outlet air temperature, cell death ranged from 3 to 7 log cycles of kill.
It must be kept in mind that pathogenic contaminants are rarely present in the biomass at the end of the fermentation period, but problems may arise during secondary treatment steps by recontamination. A preliminary heating step is also useful to induce the formation of “leached” yeast solids which aid in drying.
Products obtained by spray drying are generally lighter in color, milder in flavor, and more powdery. Drum dried products are often darker with a slightly toasted flavor which may be desirable in some uses.
The isolation of protein concentrates from biomass has been studied extensively. The first step usually consists of the mechanical disintegration of the cells to permit separation of the protoplasmic cell contents from the cell wall material. High pressure homogenizers, high speed ball mills, colloid mills, and sonic probes have been used for that purpose. Most investigators have found homogenization to be most practical. Pressures of 48,300 to 69,000 kPa (7000 to 10,000 psi) are used, and several passes through the homogenizer are usually required to obtain disintegration of more than 90% of the cells.
Disintegration of the cells is usually followed by alkaline extraction of the protein. For instance, Hedenskog et al. (1970) extracted algae and yeasts at pH 11 to 11.5, recovering 80-85% of total cell nitrogen in the extract. This was followed by precipitation of the protein at pH 4 yielding a concentrate with 70% crude protein. Alternately, Hedenskog and Mogren (1973) precipitated the protein at an alkaline pH by heating, which permitted the recovery of 60% of the original cell nitrogen in the protein precipitate.
Cunningham et al. (1975) also used alkaline extraction followed by separation from cell wall debris and precipitation at pH 3.8. They obtained a concentrate containing 70% crude protein and recovered 41.5% of the original cell nitrogen in the precipitate. All available reports from the literature led to the conclusion that one cannot hope to recover much more than 50% of the cell protein by such methods on a commercial scale.
A fraction of the cell protein is tied to the cell wall, and a large fraction of the extracted protein cannot be precipitated. Recovery of only one-half of the total cell protein doubles the raw material cost per kg (lb) of recovered protein.
This has been a major obstacle to the use of microbial biomass in food applications which require both a concentration of the protein fraction and a reduction of the NA content. A considerable improvement can be obtained by an additional processing step. If the alkaline cell extract is adjusted to a mildly acidic pH and incubated, the yeast nucleases hydrolyze nucleic acids so that they are not precipitated and carried into the protein precipitate.
Such a process has been described by Seeley (1977) for bakers’ yeast. It produces 3 fractions from the whole cell. The first fraction consists of the cell wall residue, called “glycan fraction,” which contains only 11% crude protein and 0.3% NA. This fraction has been recommended as a thickener for salad dressings because its functional properties resemble those of vegetable gums.
The second fraction contains 75% protein and only 0.7% NA, which would permit its use in various food applications without concern for the effect of NA. The third fraction contains the remaining soluble solids and has been called a “flavor fraction.” The process is probably applicable to microbial biomass other than bakers’ yeast.